Abstract
Due to the aggravation of bacterial drug resistance and the lag in the development of new antibiotics, it is crucial to develop novel therapeutic regimens for bacterial infectious diseases. Currently, immunotherapy is a promising regimen for the treatment of infectious diseases. Mucosal-associated invariant T (MAIT) cells, a subpopulation of innate-like T cells, are abundant in humans and can mount a rapid immune response to pathogens, thus becoming a potential target of immunotherapy for infectious diseases. At the site of infection, activated MAIT cells perform complex biological functions by secreting a variety of cytokines and cytotoxic substances. Many studies have shown that MAIT cells have immunoprotective effects because they can bridge innate and adaptive immune responses, leading to bacterial clearance, tissue repair, and homeostasis maintenance. MAIT cells also participate in cytokine storm generation, tissue fibrosis, and cancer progression, indicating that they play a role in immunopathology. In this article, we review recent studies of MAIT cells, discuss their dual roles in bacterial infectious diseases and provide some promising MAIT cell-targeting strategies for the treatment of bacterial infectious diseases.
Keywords: bacterial infectious disease, immunopathology, immunoprotection, immunotherapy, MAIT cells
MAIT cells can be activated in a TCR-dependent manner and/or cytokine-dependent manner. Upon activation, MAIT cells may play a protective role or pathological role in bacterial infections. Therefore, clarifying the functions of MAIT cells in bacterial diseases is a crucial step for the application of MAIT cell-targeting immunotherapies in the clinic.
Introduction
In recent years, the incidence of bacterial infectious diseases has shown an upward trend because of climate change and the aging population, making it a major public health emergency [1, 2]. Cases of multidrug-resistant bacterial infections are on the rise and becoming particularly serious and life threatening [3, 4]. Antibiotics are the first choice for the treatment of bacterial diseases; however, the emergence and spread of drug-resistant bacteria have made the treatment regimen limited in hospitalized patients due to the abuse of antibiotics [5, 6]. In addition to the administration of antibiotics, immunotherapy is another promising regimen for the treatment of bacterial diseases [7, 8]. The innate immune system is the first line of defense against pathogenic invasion, and it has the characteristic of a fast response and plays an important role in the inflammatory response to pathogenic infection [9, 10]. The second line of defense against pathogenic infection is the adaptive immune system, which can launch highly specific and strong immune responses [11, 12]. Because these two immune systems have vital roles in anti-infection [13, 14], tissue repair [15, 16], and the maintenance of homeostasis [17, 18], many researchers have been exploring their applications in many fields through targeting immune cells [19], immune molecules [20], or pathogenic bacteria [21], such as immunotherapy [22], infectious disease prevention and control [23] and disease diagnosis [24].
Innate-like T cells, a type of immune cells that acts as a bridge in the immune system, not only have the characteristics of innate immune cells but can also initiate an adaptive immune response and accelerate the immune response against invaders [25–27]. Mucosal-associated invariant T (MAIT) cells, a subpopulation of innate-like T cells discovered in 1993 [28], were named by Treiner et al.[29], and since then, the biological characteristics of MAIT cells and their roles in bacterial infectious diseases have been regularly revealed by many studies [30–33]. MAIT cells display a semi-invariant T-cell receptor (TCR) and can be activated by riboflavin-related derivatives presented by the highly conserved major histocompatibility complex class I-related molecule, MR1, in mammals [29, 34–37], implying that MAIT cells play an important role in the immune system. Moreover, MAIT cells can also be activated by cytokines, such as IL-2, IL-12, and IL-18 (Fig. 1) [38, 39].
Figure 1.
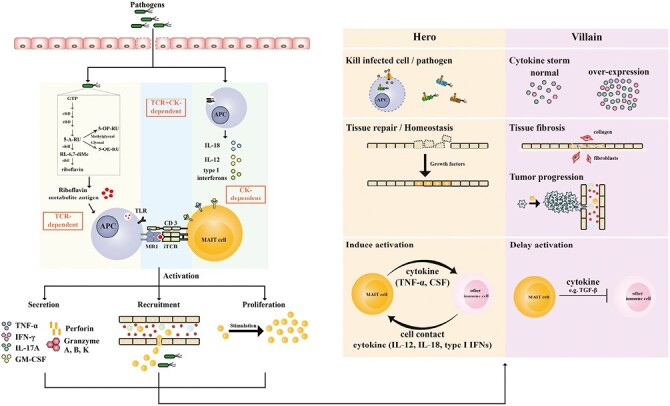
Mucosal-associated invariant T (MAIT) cells in bacterial infectious diseases. In bacterial infectious diseases, pathogens or riboflavin metabolisms are captured by antigen-presenting cells (APCs), which can activate MAIT cells in a TCR-dependent manner or cytokine (CK)-dependent manner. Upon activation, MAIT cells proliferate, secrete abundant cytokines, and then recruit and activate other immune cells, acting as a bridge to link innate and adaptive immunity. Activated MAIT cells act as heroes, villains, or both in different bacterial infectious diseases.
Upon activation, MAIT cells can initiate a rapid immune response [40, 41], produce a series of cytokines and cytotoxic substances to kill pathogens [42–47], repair impaired tissue and maintain homeostasis [48–51], launch adaptive immunity [52, 53], and trigger other immune cells to participate in immune defense (Fig. 1) [54]. Meanwhile, activated MAIT cells can also cause some adverse consequences, such as cytokine storm [55, 56], tissue fibrosis [57, 58], and the initiation and metastasis of tumor cells [59, 60]. In this review, we briefly summarize the biological characteristics of MAIT cells, focus on the roles of MAIT cells in bacterial infectious diseases, and finally discuss the therapeutic strategies targeting MAIT cells in bacterial infectious diseases and some pending questions that need to be addressed.
Biological properties of MAIT cells
Phenotype and tools
MAIT cells are abundant in the peripheral blood (1–10%) and liver (15–50%) of humans [61–63], and express an invariant TCRα chain (human: Vα7.2-Jα33; mouse: Vα19-Jα33) paired with a limited number of TCRβ chains (Vβ2, 13, and 22 in humans and Vβ6 and 8 in mice) [28, 29, 36] and many different markers or receptors correlated with the biology of MAIT cells. For example, IL-12R, IL-15R, IL-18R, and IL-23R, the cytokine receptors expressed on MAIT cells, are correlated with the cytokine-dependent activation of MAIT cells [38, 39, 64–66]; CXCR6, CCR2, CCR5, and CCR6, the tissue chemokine receptors, make that MAIT cells migrate to infected tissues [64, 67].
For our understanding of MAIT cells, the tools used to identify MAIT cells have been enriched several times, from specific TCRα primers [36] to specific antibodies (anti-Vα7.2 and anti-CD161) [68] to MR1-Ag (MR1-rRL-6-CH2OH and MR1-5-OP-RU) tetramers [69, 70]. Every new generation of tools can improve or even overcome the limitations that the last one has in identifying MAIT cells, which realizes the more accurate identification of MAIT cells. Therefore, the MR1-Ag tetramer is currently the optimized tool for identifying human and mouse MAIT cells [71, 72]. To overcome the issue that the frequency of MAIT cells is extremely low in laboratory mouse strains [73], researchers also constructed a transgenic mouse, the B6-MAITCAST mouse, in which MAIT cells are abundant, as an animal model to study MAIT cells [74]. However, this mouse strain is not widely used because of problems with transportation and customs clearance. Currently, one feasible solution to this issue is to proliferate MAIT cells by vaccinating mice before the experiment. Usually, researchers use bacteria with a riboflavin metabolic pathway and their lysates [45, 46] or 5-OP-RU with TLR agonist ligands [75] as vaccines.
Heterogeneity
MAIT cells have two main characteristics: expression of a semi-invariant TCR and restriction by the highly conserved MR1 molecule [76], implying the homogeneity of MAIT cells. However, recent studies have indicated that MAIT cells are functionally heterogeneous [77, 78]. The secretion of cytokines and cytotoxic substances is related to the distribution of MAIT cells [72, 79]. MAIT cells secrete IL-17 in the liver [80], oral [81], and female reproductive tract [82] after activation, while MAIT cells mainly secrete IFN-γ and TNF in the peripheral blood [47] and intestinal mucosa [83]. Moreover, when stimulated by Escherichia coli, MAIT cells secrete more cytokines (IFN-γ and IL-22) in the fetal small intestine than circulating MAIT cells [84], which may protect the newborn from bacterial infection. In addition, the function of MAIT cells is also related to the context. Although MAIT cells are abundant in mucosal tissues, they are not activated in healthy conditions, but are only activated after pathogenic infections [85–87]. Therefore, the activation of MAIT cells is related to the inflammatory factor environment [75, 86, 88, 89], indicating that MAIT cell function is context dependent. Furthermore, the activation way of MAIT cells is related to the time of stimulation [90]. In an in vitro experiment, Klenerman’s team found that when CD8+ T cells were cocultured with E. coli infected THP-1 cells, MAIT cells had different activation ways at different times: early activation was MR1 dependent after 5 hours of coincubation, and late activation was dependent on MR1 and IL-12 and IL-18 after 20 hours of coincubation [90]. In addition, distinct results may be obtained from the different species of animal models used to study MAIT cell function [91, 92]. Even if the species are the same, the way they are modeled could also affect the results [93, 94].
Antigens
Initially, researchers found that some bacteria could activate MAIT cells [47, 95]. Furthermore, the lysates [96, 97] and metabolites [70, 76, 98] of some bacteria can also activate MAIT cells. Moreover, some drugs and drug-like molecules also act as agonists or inhibitors of MAIT cell activation [99]. Among the metabolites of the bacteria, 5-OP-RU and 5-OE-RU [70], the derivatives of vitamin B2 (VB2), are currently known to be the two strongest agonists in activating MAIT cells. 6-FP, a photodegradation product of VB9, was the first described ligand of MR1 and plays an inhibitory role in activating MAIT cells [76]. Among drugs, diclofenac and its metabolites, methotrexate and aminopterin can activate MAIT cells [99]. Among drug-like molecules, 3-F-SA has a comparable ability to 6-FP in inhibiting the activation of MAIT cells [99]. Therefore, these drugs or drug-like molecules may also modulate the function of MAIT cells in addition to exerting their own effects when they are administered to patients.
Activation
The first described activation manner of MAIT cells is MR1-dependent manner through the MR1-TCR axis in bacterial infectious diseases [47, 76, 95]. In addition, MAIT cells can also be activated in a cytokine-dependent manner through IL-18 with IL-12, IL-15, and/or interferon α/β (Fig. 1) [38, 39, 100], which is mainly present in viral diseases [39], autoimmune diseases [58], and the diseases caused by bacteria without a riboflavin biosynthesis pathway [55]. Unsurprisingly, these two activation manners of MAIT cells are not mutually exclusive in diseases caused by some bacteria, such as Mycobacterium bovis [44] and E. coli [101], which can activate MAIT cells in a TCR- and cytokine-dependent manner. Through intranasal inoculation with riboflavin-deficient Salmonella typhimurium and/or 5-OP-OU, Chen et al. found that only inoculation with both could trigger the activation and accumulation of MAIT cells [75], indicating that the activation and accumulation of MAIT cells in vivo require the participation of both antigens and cytokines. Moreover, compared to the single manner of activation, the dual-dependent mechanism can achieve a stronger and more sustained activation of MAIT cells and enhance their cytotoxic effect [100]. Apart from antigens and/or cytokines, the activation of MAIT cells also requires secondary signals. Consistent with conventional T cells, ICOS/ICOS-L signaling is also involved in the activation and RORγt expression of MAIT cells [102]. In addition, OX40/OX40L signaling can promote the activation and proliferation of MAIT cells and then the production of IL-9—which is correlated with the severity of Helicobacter pylori-induced gastritis—by activated MAIT cells during H. pylori infection [103]. Upon activation, MAIT cells upregulate the expression of CD25 and CD69, secrete type 1 cytokines (IFN-γ and TNF-α) and/or type 17 cytokines (IL-17 and IL-22), and produce GzmB and perforin [31, 43, 45, 61, 104, 105]. These cytokines can protect the host against infection [45, 47] but can also aggravate some diseases [89, 106].
Roles of MAIT cells in bacterial infectious diseases
Protection
The first confirmed role of MAIT cells was their antibacterial function reported in 2010 [47, 95]. Subsequently, a growing number of studies suggest that mouse and human MAIT cells can play a protective role in infectious diseases caused by bacteria, such as Streptococcus pneumoniae [107], Klebsiella pneumoniae [108], Francisella tularensis [46, 109], and Legionella longbeachae [45]. In bacterial infectious diseases, MAIT cells perform antibacterial functions not only by secreting cytotoxic substances but also by recruiting and activating other immune cells [42, 54, 110, 111]. Furthermore, MAIT cells have a positive significance in the treatment of drug-resistant bacteria through secreting GzmB, perforin, and granulysin in an in vitro experiment conducted using human PBMCs [33, 112]. Moreover, an ex vivo study showed that human MAIT cells can act as an effector arm of humoral immunity against bacteria via IgG-mediated opsonization, which markedly enhances the capacity of APCs to phagocytize the target pathogen and present antigen via MR1, ultimately prompting MAIT cells to mount a strong and fast response against pathogenic infection in patients [113].
Apart from anti-bacterial properties, MAIT cells may also play a role in controlling tissue repair and homeostasis (Fig. 1) [48–51, 114, 115]. Salou et al. first proposed the hypothesis that MAIT cells are implicated in tissue repair [32], which was then proven by Constantinides et al. [51], Lamichhane et al. [50], Leng et al. [48], and Hinks et al. [49]. MAIT cells can express multiple genes that are related to tissue repair and wound healing [48–51] and possess a similar transcriptional profile as γδ T cells [116, 117] and H2-M3-restricted CD8+ T cells [49, 118], implying the tissue repair function of MAIT cells. Indeed, Leng et al. found that culture supernatants obtained from E. coli-stimulated human CD8+ MAIT cells could significantly promote the wound closure of the monolayer of colonic Caco2 cells, and this phenomenon was inhibited by an anti-MR1-blocking antibody [48]. Moreover, using experimental mouse models, Constantinides et al. found that the epidermal tongue length of the MAIT-sufficient Tcrd−/− mice topically associated with Staphylococcus epidermidis or the WT mice topically administered with 5-OP-RU was significantly longer than those observed in the MAIT-deficient Mr1−/−Tcrd−/− mice with no S. epidermidis applied or the mice with no 5-OP-RU treatment, respectively [51].
Because of their anti-infectious properties, MAIT cells can also be used as the target of vaccines or vaccine adjuvants to enhance MAIT cell-mediated protection against subsequent infections [26, 45, 102, 109, 119, 120]. MAIT cells that are enriched in the mucosa with a capacity against bacterial infections [29, 32, 104] can participate in adaptive immune responses [26, 120–122], form a stable memory-like MAIT-1 cell population in a mouse model infected with F. tularensis [109] and be boosted when mice are vaccinated with 5-OP-RU in association with different TLR agonists [75]. These properties imply that a MAIT cell-targeting vaccine can be used to boost host resistance by augmenting MAIT cell-mediated immunity. Indeed, multiple studies have proven the potential effects of MAIT cell-targeting vaccines in defending against bacterial infections. The first inspiring study investigating the role of 5-OP-RU as a “vaccine” was reported by Wang et al. [45]. They found that the mice boosted by 5-OP-RU in combination with the TLR agonist CpG or Pam2Lys had more lung MAIT cells than the naïve mice, and upon L. longbeachae challenge, the bacterial burden was significantly lower in the boosted WT mice than in the boosted MR1−/− mice, suggesting that 5-OP-RU inoculation promotes MAIT cell proliferation and then enhances host defense against bacterial infections. Furthermore, consistent with 5-OP-RU plus a TLR agonist, Wang et al. found that in a vaccinated mouse model 5-OP-RU plus IL-23 could also enhance host immunity to clear L. longbeachae [102]. Moreover, 5-OP-RU may also be used as a mucosal adjuvant to enhance bacterial vaccine efficacy through MAIT cell-mediated B-cell help [26, 120, 121]. Jensen et al. investigated the ability of 5-OP-RU as the adjuvant of a Vibrio cholerae O1 polysaccharide conjugate vaccine in a mouse model and found that mice could produce a higher level of mucosal IgG antibodies in the 5-A-RU plus methylglyoxal group than in the methylglyoxal alone group, protecting vaccinated mice from subsequent V. cholera challenge [120].
MAIT cells, as an innate-like T-cell subpopulation, can bridge innate and adaptive immunity. After activation by bacteria, MAIT cells secrete multiple cytokines to crosstalk with monocytes, dendritic cells (DCs), B cells, and neutrophils and then mount and/or fine tune immune responses [26, 54, 123, 124]. Mouse MAIT cells activated by F. tularensis LVS can promote the early production of pulmonary GM-CSF, which is necessary for the differentiation of inflammatory monocytes into monocyte-derived DCs (Mo-DCs), and Mo-DCs trigger the activation and accumulation of CD4+ T cells in the lungs [54]. In addition, human MAIT cells activated by the cognate Ag 5-A-RU/MG can upregulate the expression of CD40L and induce CD40L- and MR1-dependent DC maturation, and DCs then upregulate IL-12 expression, which in turn promotes MAIT cell activation and provides help to efficiently prime CTLs [123]. Microbe-activated human MAIT cells can promote plasmablast formation and antibody production by secreting B-cell stimulatory factors in in vitro experiments [121]. Furthermore, Jensen et al. reported that the adaptive transfer of CXCR5+ T follicular helper-like MAIT cells into αβ T-cell deficient mice enhances host humoral immune responses to V. cholerae by expressing B-cell helper cytokines (IL-21, IL-10, and BAFF), indicating a protective role of mouse MAIT cells against V. cholerae infection [26]. Moreover, the mutual restriction between MAIT cells and neutrophils may be present in humans to balance immune responses [124]. Although activated MAIT cells can recruit neutrophils to augment immune responses [50], strongly activated MAIT cells induce neutrophil death by producing a high level of TNF-α; in turn, neutrophils inhibit the overactivation of MAIT cells in a cell-contact and ROS-dependent manner [124], suggesting a negative regulatory feedback mechanism to protect the host from excessive inflammation and respond to bacterial infection.
Pathology
Although many studies have reported the protective function of MAIT cells against bacterial infections, their pathogenic role exists in some settings (Supplementary Table S1). Upon activation by superantigens (SAgs), human MAIT cells launch a robust proinflammatory cytokine response by producing large amounts of IFN-γ and TNF-α, which may lead to a systemic cytokine storm and could be life-threatening for the host [55, 56]. Moreover, in a H. pylori infection model, D’souza et al. found that 5-OP-RU-treated mice displayed more serious gastric pathology than mice without MAIT cell expansion, suggesting that MAIT cells may be implicated in the pathological process of gastritis caused by H. pylori [89]. This mechanism may be related to MAIT cell-secreted IFN-γ, TNF-α, and IL-17A [89, 125].
Recently, several reports demonstrated that MAIT cells are involved in tissue repair and homeostasis when activated by commensal bacteria or pathogenic bacteria, which is a novel role of MAIT cells in maintaining host health [48–51]. However, it has also been reported that MAIT cells executing an improper tissue repair function may lead to tissue fibrosis in the liver [57, 58] and kidney [126] and may also promote tumor growth by expressing tissue repair- and/or angiogenesis-related genes [41, 114]. In patients with alcoholic liver diseases, if intestinal barrier integrity is damaged, liver MAIT cells can be activated by bacteria from the gut and then secrete cytokines and cytotoxic substances [127, 128], aggravating liver inflammation and fibrosis [129, 130]. In addition, MR1 can be expressed in many cells [131], which means that they can activate MAIT cells using agonist ligands. Although MAIT cell antigens in cancer have not yet been identified, the existence of bacteria inside or on the surface of the tumor has been verified [132]; therefore, bacterial antigens presented by tumor cells can activate and recruit MAIT cells, which has been proven to have protumor effects [59, 133].
Researchers also found that MAIT cells could suppress host immune responses by communicating with other immune cells in certain bacterial infectious diseases [42, 54, 110, 111]. Sakai et al. found that in an experimental mouse model, the activated MAIT cells of mice vaccinated with 5-OP-RU + CpG inhibited CCR2+Ly6C+ myeloid cells trafficking into the mLNs in a TGF-β-dependent manner, which delayed the priming of Mycobacterium tuberculosis-specific CD4+ T cells and affected the adaptive immune response [93]. Moreover, in the S. typhimurium BRD509 or Pam2Cys plus 5-OP-RU preprimed mouse model, activated MAIT cells recruited immune cells, including neutrophils, macrophages, eosinophils, and dendritic cells, into the H. pylori-infected gastric mucosa, which aggravated gastritis [89, 134]. In addition, in some tissues, MAIT cells can recruit neutrophils into the infected site through the secretion of IL-17 [135, 136]. These recruited neutrophils may exacerbate tissue inflammation by secreting a panel of cytokines and cytotoxic substances [137, 138]. Importantly, in research on tumor immunology, MAIT cells can suppress the anti-tumor function of NK cells and enhance the permeability of the tissue mucosal and/or vascular barrier [59, 139], which results in the translocation of flora [140], aggravates the inflammatory response, and promotes tumor metastasis.
MAIT cell-targeting strategies for antibacterial therapy
As a subpopulation of innate-like T cells, MAIT cells can launch a rapid, non-specific innate immune response and mount a specific adaptive immune response upon activation, endowing them with a versatile function in antibacterial infectious diseases [25, 54]. Noteworthily, the function(s) of MAIT cells in bacterial diseases must be taken into consideration when MAIT cell-targeting strategies are used to treat these diseases [45, 61, 89].
Given the excellent protective role of MAIT cells in bacterial infectious diseases, MAIT cells can be considered a therapeutic target through priming and expanding them by using agonist ligands or vaccine strains [93, 120, 141, 142]. First, MAIT cells display a good protective effect against infections by intractable bacteria, such as superbacteria [112]. Second, MAIT cells can also be used as the target of vaccine adjuvants to enhance bacterial vaccine efficacy [26, 45, 120]. Third, based on the immune checkpoints expressed on the surface of MAIT cells [111, 143, 144], MAIT cells can also act as targets to treat infectious diseases [145, 146]. PD-1, a well-known immune checkpoint [147], is also expressed on MAIT cells [88, 144, 145]. In patients with active tuberculosis, the expression of PD-1 on MAIT cells in pleural effusion was high and correlated with the deficiency of IFN-γ [144], and the secretion of IFN-γ was restored to exert anti-bacterial effects by using purified PD-1 antibody, implying that PD-1 might be a new therapeutic target of bacterial infectious diseases [144].
Given that MAIT cells also play a pathological role in some diseases, inhibiting MAIT cell activation or neutralizing the cytokines and cytotoxic substances secreted by MAIT cells may also be a new therapeutic strategy. Superantigens that are expressed by some bacteria can activate MAIT cells and cause a cytokine storm [55, 56]. The inhibitor ligands of MAIT cells, such as 6-FP [148] and Ac-6-FP [149], can be taken into consideration in the treatment of diseases. This strategy has been studied in the treatment of tumors [59], inflammatory bowel disease [150], and so on. In addition, Barber’s team found that the activation of MAIT cells delayed the priming of Mtb-specific CD4+ T cells, which indicates that using an inhibitor ligand may be a candidate strategy for tuberculosis treatment [93]. Furthermore, in patients infected with H. pylori [89], IL-17A neutralizing antibody may be a good choice to alleviate the pathological responses caused by MAIT cells, such as gastric mucosal injury and gastritis.
Concluding remarks
Since MAIT cells were first discovered in 1993, they have become a star player in the field of T-cell immunity due to their nonredundant functions in the immune system. In the past several decades, the enigmatic veils of MAIT cells in phenotype, antigen, activation, and development have been revealed. MAIT cells are a double-edged sword, unfortunately, and how to avoid their pathogenicity when using the protective role of MAIT cells is the most important aspect of MAIT cell research.
Although the role of MAIT cells in some diseases has been clarified, further studies are needed to comprehensively elucidate the functions of MAIT cells in bacterial infectious diseases. First, based on their location, MAIT cells are divided into two categories, peripheral blood-circulatory MAIT cells and tissue-resident MAIT cells. However, the relationship between these two groups and whether the conclusions can be extended to each other are not clear. It has been reported that the number and molecular phenotype of MAIT cells in peripheral blood are different from those of tissue-resident MAIT cells, and their responses to pathogenic bacteria are also different [72, 79]; therefore, further studies are needed to investigate these two groups of MAIT cells. Second, although there is abundant commensal flora in the gastrointestinal tract, some of these bacteria have a riboflavin metabolic pathway that can produce riboflavin metabolic derivatives that activate MAIT cells. In an in vivo environment, MAIT cells are not continuously activated to produce excessive cytokines, but only when they are infected by pathogenic bacteria are MAIT cells activated to exert antibacterial effects [87]. However, the definite mechanism of this condition is still unclear. Third, the current studies on the antibacterial function of human MAIT cells have mainly focused on PBMC-derived MAIT cells. The role and mechanism of MAIT cells in response to bacterial infectious diseases in humans are still not clear, which is not conducive to the clinical application of MAIT cells. Based on the existing conclusions, we found that the number of MAIT cells in the infection site is often increased and that the increased MAIT cells can secrete antibacterial cytokines; therefore, MAIT cells can be used as a new target for antibacterial therapy [42, 45, 93, 108].
For bacterial infectious diseases, treatment is still a major challenge due to the abuse of antibiotics, climate change, and rising population mobility. In addition to the development of new antibiotics, immunotherapy is another promising candidate for the treatment of bacterial diseases. Many studies have suggested that MAIT cells can play important roles in bacterial infectious diseases. However, MAIT cells not only act as heroes but also as villains in some diseases. Therefore, clarifying the functions of MAIT cells in the progression of diseases and formulating a personalized therapeutic scheme is a crucial step for the application of MAIT cell-targeting immunotherapies in the clinic.
Supplementary Material
Glossary
Abbreviations
- 3-F-SA
3-formylsalicylic acid
- 5-A-RU
5-amino-6-D-ribitylaminouracil
- 5-OE-RU
5-(2-oxoethylideneamino)-6-D-ribitylaminouracil
- 5-OP-RU
5-(2-oxopropylideneamino)-6-D-ribitylaminouracil
- 6-FP
6-formylpterin
- Ac-6-FP
acetyl-6-formylpterin
- APC
antigen-presenting cell
- BAFF
B-cell activating factor
- CTL
cytotoxic T cells
- DC
dendritic cell
- GM-CSF
granulocyte-macrophage colony-stimulating factor
- ICOS
inducible costimulator
- IFN
interferon
- MR1
major histocompatibility complex class I-related protein 1
- MAIT
mucosal-associated invariant T
- PBMC
peripheral blood mononuclear cell
- ROS
reactive oxygen species
- rRL-6-CH2OH
reduced 6-hydroxymethyl-8-D-ribityllumazine
- TCR
T-cell receptor
- THP-1
Tohoku Hospital Pediatrics-1
- TLR
Toll-like receptor
- TNF
tumor necrosis factor
- TGF
transforming growth factor
Contributor Information
Sihong Wu, Wenzhou Key Laboratory of Sanitary Microbiology, Key Laboratory of Laboratory Medicine, Ministry of Education, School of Laboratory Medicine and Life Sciences, Wenzhou Medical University, Wenzhou, China.
Xi Yang, Wenzhou Key Laboratory of Sanitary Microbiology, Key Laboratory of Laboratory Medicine, Ministry of Education, School of Laboratory Medicine and Life Sciences, Wenzhou Medical University, Wenzhou, China.
Yongliang Lou, Wenzhou Key Laboratory of Sanitary Microbiology, Key Laboratory of Laboratory Medicine, Ministry of Education, School of Laboratory Medicine and Life Sciences, Wenzhou Medical University, Wenzhou, China.
Xingxing Xiao, Wenzhou Key Laboratory of Sanitary Microbiology, Key Laboratory of Laboratory Medicine, Ministry of Education, School of Laboratory Medicine and Life Sciences, Wenzhou Medical University, Wenzhou, China.
Funding
This work was supported by the National Natural Science Foundation of China (grant no. 82002117), the Scientific Research Project of Wenzhou Medical University (grant no. XY2022002), and the Key Discipline of Zhejiang Province in Medical Technology (First Class, Category A).
Conflict of interests
The authors declare no conflicts of interest.
Author contributions
All authors contributed to the conception, writing, and revision of this article.
References
- 1. Collaborators GDaIIaP. Global, regional, and national incidence, prevalence, and years lived with disability for 354 diseases and injuries for 195 countries and territories, 1990-2017: a systematic analysis for the Global Burden of Disease Study 2017. Lancet 2018, 392, 1789–858. doi: 10.1016/S0140-6736(18)32279-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Collaborators GDaI. Global burden of 369 diseases and injuries in 204 countries and territories, 1990-2019: a systematic analysis for the Global Burden of Disease Study 2019. Lancet 2020, 396, 1204–22. doi: 10.1016/S0140-6736(20)30925-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Furuya EY, Lowy FD.. Antimicrobial-resistant bacteria in the community setting. Nat Rev Microbiol 2006, 4, 36–45. doi: 10.1038/nrmicro1325 [DOI] [PubMed] [Google Scholar]
- 4. Tang SS, Apisarnthanarak A, Hsu LY.. Mechanisms of β-lactam antimicrobial resistance and epidemiology of major community- and healthcare-associated multidrug-resistant bacteria. Adv Drug Deliv Rev 2014, 78, 3–13. doi: 10.1016/j.addr.2014.08.003 [DOI] [PubMed] [Google Scholar]
- 5. Jernigan JA, Hatfield KM, Wolford H, Nelson RE, Olubajo B, Reddy SC, et al. Multidrug-resistant bacterial infections in U.S. hospitalized patients, 2012-2017. N Engl J Med 2020, 382, 1309–19. doi: 10.1056/NEJMoa1914433 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Bhattarai S, Sharma BK, Subedi N, Ranabhat S, Baral MP.. Burden of serious bacterial infections and multidrug-resistant organisms in an adult population of Nepal: a comparative analysis of minimally invasive tissue sampling informed mortality surveillance of community and hospital deaths. Clin Inf Dis 2021, 73, S415–21. doi: 10.1093/cid/ciab773 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Hancock RE, Nijnik A, Philpott DJ.. Modulating immunity as a therapy for bacterial infections. Nat Rev Microbiol 2012, 10, 243–54. doi: 10.1038/nrmicro2745 [DOI] [PubMed] [Google Scholar]
- 8. Dittmer U, Olbrich AR.. Treatment of infectious diseases with immunostimulatory oligodeoxynucleotides containing CpG motifs. Curr Opin Microbiol 2003, 6, 472–7. doi: 10.1016/j.mib.2003.09.007 [DOI] [PubMed] [Google Scholar]
- 9. Thaiss CA, Zmora N, Levy M, Elinav E.. The microbiome and innate immunity. Nature 2016, 535, 65–74. doi: 10.1038/nature18847 [DOI] [PubMed] [Google Scholar]
- 10. Brubaker SW, Bonham KS, Zanoni I, Kagan JC.. Innate immune pattern recognition: a cell biological perspective. Annu Rev Immunol 2015, 33, 257–90. doi: 10.1146/annurev-immunol-032414-112240 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Clegg J, Soldaini E, Bagnoli F, McLoughlin RM.. Targeting skin-resident memory T cells via vaccination to combat Staphylococcus aureus infections. Trends Immunol 2021, 42, 6–17. doi: 10.1016/j.it.2020.11.005 [DOI] [PubMed] [Google Scholar]
- 12. Bonilla FA, Oettgen HC.. Adaptive immunity. J Allergy Clin Immunol 2010, 125, S33–40. doi: 10.1016/j.jaci.2009.09.017 [DOI] [PubMed] [Google Scholar]
- 13. Albiger B, Dahlberg S, Henriques-Normark B, Normark S.. Role of the innate immune system in host defence against bacterial infections: focus on the Toll-like receptors. J Intern Med 2007, 261, 511–28. doi: 10.1111/j.1365-2796.2007.01821.x [DOI] [PubMed] [Google Scholar]
- 14. Bröker BM, Mrochen D, Péton V.. The T Cell response to Staphylococcus aureus. Pathogens 2016, 5, 31. doi: 10.3390/pathogens5010031 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Soliman AM, Barreda DR.. Acute inflammation in tissue healing. Int J Mol Sci 2022, 24, 641. doi: 10.3390/ijms24010641 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Planer JD, Morrisey EE.. After the storm: regeneration, repair, and reestablishment of homeostasis between the alveolar epithelium and innate immune system following viral lung injury. Annu Rev Pathol 2023, 18, 337–59. doi: 10.1146/annurev-pathmechdis-031621-024344 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. LeBlanc G, Kreissl FK, Melamed J, Sobel AL, Constantinides MG.. The role of unconventional T cells in maintaining tissue homeostasis. Semin Immunol 2022, 61-64, 101656. doi: 10.1016/j.smim.2022.101656 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Chovatiya R, Medzhitov R.. Stress, inflammation, and defense of homeostasis. Mol Cell 2014, 54, 281–8. doi: 10.1016/j.molcel.2014.03.030 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Koch MR, Gong R, Friedrich V, Engelsberger V, Kretschmer L, Wanisch A, et al. CagA-specific gastric CD8(+) tissue-resident T cells control Helicobacter pylori during the early infection phase. Gastroenterology 2022, 164, 550–566. doi: 10.1053/j.gastro.2022.12.016 [DOI] [PubMed] [Google Scholar]
- 20. Feng Y, Chen Z, Tu SQ, Wei JM, Hou YL, Kuang ZL, et al. Role of interleukin-17A in the pathomechanisms of periodontitis and related systemic chronic inflammatory diseases. Front Immunol 2022, 13, 862415. doi: 10.3389/fimmu.2022.862415 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Perez-Lopez A, Behnsen J, Nuccio SP, Raffatellu M.. Mucosal immunity to pathogenic intestinal bacteria. Nat Rev Immunol 2016, 16, 135–48. doi: 10.1038/nri.2015.17 [DOI] [PubMed] [Google Scholar]
- 22. Huang X, Pan J, Xu F, Shao B, Wang Y, Guo X, et al. Bacteria-Based Cancer Immunotherapy. Adv Sci (Weinh) 2021, 8, 2003572. doi: 10.1002/advs.202003572 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Curtiss R 3rd. Bacterial infectious disease control by vaccine development. J Clin Invest 2002, 110, 1061–6. doi: 10.1172/JCI16941 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Yang S, Liu T, Cheng Y, Bai Y, Liang G.. Immune cell infiltration as a biomarker for the diagnosis and prognosis of digestive system cancer. Cancer Sci 2019, 110, 3639–49. doi: 10.1111/cas.14216 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Huang S. Targeting innate-like T cells in tuberculosis. Front Immunol 2016, 7, 594. doi: 10.3389/fimmu.2016.00594 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Jensen O, Trivedi S, Meier JD, Fairfax KC, Hale JS, Leung DT.. A subset of follicular helper-like MAIT cells can provide B cell help and support antibody production in the mucosa. Sci Immunol 2022, 7, eabe8931. doi: 10.1126/sciimmunol.abe8931 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Mayassi T, Barreiro LB, Rossjohn J, Jabri B.. A multilayered immune system through the lens of unconventional T cells. Nature 2021, 595, 501–10. doi: 10.1038/s41586-021-03578-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Porcelli S, Yockey CE, Brenner MB, Balk SP.. Analysis of T cell antigen receptor (TCR) expression by human peripheral blood CD4-8- alpha/beta T cells demonstrates preferential use of several V beta genes and an invariant TCR alpha chain. J Exp Med 1993, 178, 1–16. doi: 10.1084/jem.178.1.1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Emmanuel T, Livine D, Seiamak B, Mirjana R, Valerie W, Florence T, et al. Selection of evolutionarily conserved mucosal-associated invariant T cells by MR1. Nature 2003, 422, 164–169. doi: 10.1038/nature01433 [DOI] [PubMed] [Google Scholar]
- 30. Le Bourhis L, Guerri L, Dusseaux M, Martin E, Soudais C, Lantz O.. Mucosal-associated invariant T cells: unconventional development and function. Trends Immunol 2011, 32, 212–8. doi: 10.1016/j.it.2011.02.005 [DOI] [PubMed] [Google Scholar]
- 31. Franciszkiewicz K, Salou M, Legoux F, Zhou Q, Cui Y, Bessoles S, et al. MHC class I-related molecule, MR1, and mucosal-associated invariant T cells. Immunol Rev 2016, 272, 120–38. doi: 10.1111/imr.12423 [DOI] [PubMed] [Google Scholar]
- 32. Salou M, Franciszkiewicz K, Lantz O.. MAIT cells in infectious diseases. Curr Opin Immunol 2017, 48, 7–14. doi: 10.1016/j.coi.2017.07.009 [DOI] [PubMed] [Google Scholar]
- 33. Leeansyah E, Boulouis C, Kwa ALH, Sandberg JK.. Emerging role for MAIT cells in control of antimicrobial resistance. Trends Microbiol 2021, 29, 504–16. doi: 10.1016/j.tim.2020.11.008 [DOI] [PubMed] [Google Scholar]
- 34. Huang S, Gilfillan S, Cella M, Miley MJ, Lantz O, Lybarger L, et al. Evidence for MR1 antigen presentation to mucosal-associated invariant T cells. J Biol Chem 2005, 280, 21183–93. doi: 10.1074/jbc.M501087200 [DOI] [PubMed] [Google Scholar]
- 35. Boudinot P, Mondot S, Jouneau L, Teyton L, Lefranc MP, Lantz O.. Restricting nonclassical MHC genes coevolve with TRAV genes used by innate-like T cells in mammals. Proc Natl Acad Sci U S A 2016, 113, E2983–92. doi: 10.1073/pnas.1600674113 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Tilloy F, Treiner E, Park S, Garcia C, Lemonnier F, Salle H, et al. An invariant T cell receptor a chain define a novel TAP-independent major histocompatibility complex class Ib-restricted a/b T cell subpopulation in mammals. J Exp Med 189. J Exp Med 1999, 189, 1907–21. doi: 10.1084/jem.189.12.1907 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Riegert P, Wanner V, Bahram S.. Genomics, isoforms, expression, and phylogeny of the MHC class I-related MR1 gene. J Immunol 1998, 161, 4066–77. doi: 10.4049/jimmunol.161.8.4066 [DOI] [PubMed] [Google Scholar]
- 38. Ussher JE, Bilton M, Attwod E, Shadwell J, Richardson R, de Lara C, et al. CD161++ CD8+ T cells, including the MAIT cell subset, are specifically activated by IL-12+IL-18 in a TCR-independent manner. Eur J Immunol 2014, 44, 195–203. doi: 10.1002/eji.201343509 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. van Wilgenburg B, Scherwitzl I, Hutchinson EC, Leng T, Kurioka A, Kulicke C, et al. MAIT cells are activated during human viral infections. Nat Commun 2016, 7, 11653. doi: 10.1038/ncomms11653 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Shouxiong H. Targeting innate-like T cells in tuberculosis. Front Immunol 2016, 7, 594. doi: 10.3389/fimmu.2016.00594 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Legoux F, Salou M, Lantz O.. MAIT cell development and functions: the microbial connection. Immunity 2020, 53, 710–23. doi: 10.1016/j.immuni.2020.09.009 [DOI] [PubMed] [Google Scholar]
- 42. Toubal A, Nel I, Lotersztajn S, Lehuen A.. Mucosal-associated invariant T cells and disease. Nat Rev Immunol 2019, 19, 643–57. doi: 10.1038/s41577-019-0191-y [DOI] [PubMed] [Google Scholar]
- 43. Kurioka A, Ussher JE, Cosgrove C, Clough C, Fergusson JR, Smith K, et al. MAIT cells are licensed through granzyme exchange to kill bacterially sensitized targets. Mucosal Immunol 2015, 8, 429–40. doi: 10.1038/mi.2014.81 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Chua WJ, Truscott SM, Eickhoff CS, Blazevic A, Hoft DF, Hansen TH.. Polyclonal mucosa-associated invariant T cells have unique innate functions in bacterial infection. Infect Immun 2012, 80, 3256–67. doi: 10.1128/IAI.00279-12 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Wang H, D’Souza C, Lim XY, Kostenko L, Pediongco TJ, Eckle SBG, et al. MAIT cells protect against pulmonary Legionella longbeachae infection. Nat Commun 2018, 9, 3350. doi: 10.1038/s41467-018-05202-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Meierovics A, Yankelevich WJ, Cowley SC.. MAIT cells are critical for optimal mucosal immune responses during in vivo pulmonary bacterial infection. Proc Natl Acad Sci U S A 2013, 110, E3119–28. doi: 10.1073/pnas.1302799110 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Le Bourhis L, Martin E, Péguillet I, Guihot A, Froux N, Coré M, et al. Antimicrobial activity of mucosal-associated invariant T cells. Nat Immunol 2010, 11, 701–8. doi: 10.1038/ni.1890 [DOI] [PubMed] [Google Scholar]
- 48. Leng T, Akther HD, Hackstein CP, Powell K, King T, Friedrich M, et al. TCR and inflammatory signals tune human MAIT cells to exert specific tissue repair and effector functions. Cell Rep 2019, 28, 3077–3091.e5. doi: 10.1016/j.celrep.2019.08.050 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Hinks TSC, Marchi E, Jabeen M, Olshansky M, Kurioka A, Pediongco TJ, et al. Activation and in vivo evolution of the MAIT cell transcriptome in mice and humans reveals tissue repair functionality. Cell Rep 2019, 28, 3249–3262.e5. doi: 10.1016/j.celrep.2019.07.039 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Lamichhane R, Schneider M, de la Harpe SM, Harrop TWR, Hannaway RF, Dearden PK, et al. TCR- or cytokine-activated CD8(+) mucosal-associated invariant T cells are rapid polyfunctional effectors that can coordinate immune responses. Cell Rep 2019, 28, 3061–3076.e5. doi: 10.1016/j.celrep.2019.08.054 [DOI] [PubMed] [Google Scholar]
- 51. Constantinides MG, Link VM, Tamoutounour S, Wong AC, Perez-Chaparro PJ, Han SJ, et al. MAIT cells are imprinted by the microbiota in early life and promote tissue repair. Science 2019, 366, eaax6624. doi: 10.1126/science.aax6624 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Salio M. Unconventional MAIT cell responses to bacterial infections. Semin Immunol 2022, 61–64, 101663. doi: 10.1016/j.smim.2022.101663 [DOI] [PubMed] [Google Scholar]
- 53. Su B, Kong D, Yang X, Zhang T, Kuang YQ.. Mucosal-associated invariant T cells: A cryptic coordinator in HIV-infected immune reconstitution. J Med Virol 2022, 94, 3043–53. doi: 10.1002/jmv.27696 [DOI] [PubMed] [Google Scholar]
- 54. Meierovics AI, Cowley SC.. MAIT cells promote inflammatory monocyte differentiation into dendritic cells during pulmonary intracellular infection. J Exp Med 2016, 213, 2793–809. doi: 10.1084/jem.20160637 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Emgård J, Bergsten H, McCormick JK, Barrantes I, Skrede S, Sandberg JK, et al. MAIT cells are major contributors to the cytokine response in group A streptococcal toxic shock syndrome. Proc Natl Acad Sci USA 2019, 116, 25923–31. doi: 10.1073/pnas.1910883116 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Shaler CR, Choi J, Rudak PT, Memarnejadian A, Szabo PA, Tun-Abraham ME, et al. MAIT cells launch a rapid, robust and distinct hyperinflammatory response to bacterial superantigens and quickly acquire an anergic phenotype that impedes their cognate antimicrobial function: defining a novel mechanism of superantigen-induced immunopathology and immunosuppression. PLoS Biol 2017, 15, e2001930. doi: 10.1371/journal.pbio.2001930 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Hegde P, Weiss E, Paradis V, Wan J, Mabire M, Sukriti S, et al. Mucosal-associated invariant T cells are a profibrogenic immune cell population in the liver. Nat Commun 2018, 9, 2146. doi: 10.1038/s41467-018-04450-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Böttcher K, Rombouts K, Saffioti F, Roccarina D, Rosselli M, Hall A, et al. MAIT cells are chronically activated in patients with autoimmune liver disease and promote profibrogenic hepatic stellate cell activation. Hepatology 2018, 68, 172–86. doi: 10.1002/hep.29782 [DOI] [PubMed] [Google Scholar]
- 59. Yan J, Allen S, McDonald E, Das I, Mak JYW, Liu L, et al. MAIT cells promote tumor initiation, growth, and metastases via tumor MR1. Cancer Discov 2020, 10, 124–41. doi: 10.1158/2159-8290.CD-19-0569 [DOI] [PubMed] [Google Scholar]
- 60. Petley EV, Koay HF, Henderson MA, Sek K, Todd KL, Keam SP, et al. MAIT cells regulate NK cell-mediated tumor immunity. Nat Commun 2021, 12, 4746. doi: 10.1038/s41467-021-25009-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Provine NM, Klenerman P.. MAIT cells in health and disease. Annu Rev Immunol 2020, 38, 203–28. doi: 10.1146/annurev-immunol-080719-015428 [DOI] [PubMed] [Google Scholar]
- 62. Kurioka A, Walker LJ, Klenerman P, Willberg CB.. MAIT cells: new guardians of the liver. Clin Transl Immunol 2016, 5, e98. doi: 10.1038/cti.2016.51 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Treiner E, Lantz O.. CD1d- and MR1-restricted invariant T cells: of mice and men. Curr Opin Immunol 2006, 18, 519–26. doi: 10.1016/j.coi.2006.07.001 [DOI] [PubMed] [Google Scholar]
- 64. Billerbeck E, Kang YH, Walker L, Lockstone H, Grafmueller S, Fleming V, et al. Analysis of CD161 expression on human CD8+ T cells defines a distinct functional subset with tissue-homing properties. Proc Natl Acad Sci U S A 2010, 107, 3006–11. doi: 10.1073/pnas.0914839107 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Jesteadt E, Zhang I, Yu H, Meierovics A, Chua Yankelevich WJ, Cowley S.. Interleukin-18 is critical for mucosa-associated invariant T cell gamma interferon responses to francisella species in vitro but not in vivo. Infect Immun 2018, 86, e00117–18. doi: 10.1128/IAI.00117-18 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Sortino O, Richards E, Dias J, Leeansyah E, Sandberg JK, Sereti I.. IL-7 treatment supports CD8+ mucosa-associated invariant T-cell restoration in HIV-1-infected patients on antiretroviral therapy. AIDS 2018, 32, 825–8. doi: 10.1097/QAD.0000000000001760 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. Voillet V, Buggert M, Slichter CK, Berkson JD, Mair F, Addison MM, et al. Human MAIT cells exit peripheral tissues and recirculate via lymph in steady state conditions. JCI Insight 2018, 3, e98487. doi: 10.1172/jci.insight.98487 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. Martin E, Treiner E, Duban L, Guerri L, Laude H, Toly C, et al. Stepwise Development of MAIT cells in mouse and human. PLoS Biol 2009, 7, e54. doi: 10.1371/journal.pbio.1000054 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69. Reantragoon R, Corbett AJ, Sakala IG, Gherardin NA, Furness JB, Chen Z, et al. Antigen-loaded MR1 tetramers define T cell receptor heterogeneity in mucosal-associated invariant T cells. J Exp Med 2013, 210, 2305–20. doi: 10.1084/jem.20130958 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70. Corbett AJ, Eckle SB, Birkinshaw RW, Liu L, Patel O, Mahony J, et al. T-cell activation by transitory neo-antigens derived from distinct microbial pathways. Nature 2014, 509, 361–5. doi: 10.1038/nature13160. [DOI] [PubMed] [Google Scholar]
- 71. Gherardin NA, Souter MN, Koay HF, Mangas KM, Seemann T, Stinear TP, et al. Human blood MAIT cell subsets defined using MR1 tetramers. Immunol Cell Biol 2018, 96, 507–25. doi: 10.1111/imcb.12021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72. Rahimpour A, Koay HF, Enders A, Clanchy R, Eckle SB, Meehan B, et al. Identification of phenotypically and functionally heterogeneous mouse mucosal-associated invariant T cells using MR1 tetramers. J Exp Med 2015, 212, 1095–108. doi: 10.1084/jem.20142110 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73. Tilloy F, Treiner E, Park SH, Garcia C, Lemonnier F, de la Salle H, et al. An invariant T cell receptor alpha chain defines a novel TAP-independent major histocompatibility complex class Ib-restricted alpha/beta T cell subpopulation in mammals. J Exp Med 1999, 189, 1907–21. doi: 10.1084/jem.189.12.1907 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74. Cui Y, Franciszkiewicz K, Mburu YK, Mondot S, Le Bourhis L, Premel V, et al. Mucosal-associated invariant T cell-rich congenic mouse strain allows functional evaluation. J Clin Invest 2015, 125, 4171–85. doi: 10.1172/JCI82424 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75. Chen Z, Wang H, D’Souza C, Sun S, Kostenko L, Eckle SB, et al. Mucosal-associated invariant T-cell activation and accumulation after in vivo infection depends on microbial riboflavin synthesis and co-stimulatory signals. Mucosal Immunol 2017, 10, 58–68. doi: 10.1038/mi.2016.39 [DOI] [PubMed] [Google Scholar]
- 76. Kjer-Nielsen L, Patel O, Corbett AJ, Le Nours J, Meehan B, Liu L, et al. MR1 presents microbial vitamin B metabolites to MAIT cells. Nature 2012, 491, 717–23. doi: 10.1038/nature11605 [DOI] [PubMed] [Google Scholar]
- 77. Dias J, Leeansyah E, Sandberg JK.. Multiple layers of heterogeneity and subset diversity in human MAIT cell responses to distinct microorganisms and to innate cytokines. Proc Natl Acad Sci U S A 2017, 114, E5434–43. doi: 10.1073/pnas.1705759114 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78. Dias J, Boulouis C, Sobkowiak MJ, Lal KG, Emgård J, Buggert M, et al. Factors Influencing functional heterogeneity in human mucosa-associated invariant T cells. Front Immunol 2018, 9, 1602. doi: 10.3389/fimmu.2018.01602 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79. Solders M, Gorchs L, Erkers T, Lundell A-C, Nava S, Gidlöf S, et al. MAIT cells accumulate in placental intervillous space and display a highly cytotoxic phenotype upon bacterial stimulation. Sci Rep 2017, 7, 6123. doi: 10.1038/s41598-017-06430-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80. Tang XZ, Jo J, Tan AT, Sandalova E, Chia A, Tan KC, et al. IL-7 licenses activation of human liver intrasinusoidal mucosal-associated invariant T cells. J Immunol 2013, 190, 3142–52. doi: 10.4049/jimmunol.1203218 [DOI] [PubMed] [Google Scholar]
- 81. Sobkowiak MJ, Davanian H, Heymann R, Gibbs A, Emgård J, Dias J, et al. Tissue-resident MAIT cell populations in human oral mucosa exhibit an activated profile and produce IL-17. Eur J Immunol 2019, 49, 133–43. doi: 10.1002/eji.201847759 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82. Gibbs A, Leeansyah E, Introini A, Paquin-Proulx D, Hasselrot K, Andersson E, et al. MAIT cells reside in the female genital mucosa and are biased towards IL-17 and IL-22 production in response to bacterial stimulation. Mucosal Immunol 2017, 10, 35–45. doi: 10.1038/mi.2016.30 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83. Gao MG, Hong Y, Zhao XY, Pan XA, Sun YQ, Kong J, et al. The potential roles of mucosa-associated invariant T cells in the pathogenesis of gut graft-versus-host disease after hematopoietic stem cell transplantation. Front Immunol 2021, 12, 720354. doi: 10.3389/fimmu.2021.720354 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84. Leeansyah E, Loh L, Nixon DF, Sandberg JK.. Acquisition of innate-like microbial reactivity in mucosal tissues during human fetal MAIT-cell development. Nat Commun 2014, 5, 3143. doi: 10.1038/ncomms4143 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85. McWilliam HEG, Villadangos JA.. How MR1 presents a pathogen metabolic signature to mucosal-associated invariant T (MAIT) cells. Trends Immunol 2017, 38, 679–89. doi: 10.1016/j.it.2017.06.005 [DOI] [PubMed] [Google Scholar]
- 86. Slichter CK, McDavid A, Miller HW, Finak G, Seymour BJ, McNevin JP, et al. Distinct activation thresholds of human conventional and innate-like memory T cells. JCI Insight 2016, 1, e86292. doi: 10.1172/jci.insight.86292 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87. Tastan C, Karhan E, Zhou W, Fleming E, Voigt AY, Yao X, et al. Tuning of human MAIT cell activation by commensal bacteria species and MR1-dependent T-cell presentation. Mucosal Immunol 2018, 11, 1591–605. doi: 10.1038/s41385-018-0072-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88. Nel I, Bertrand L, Toubal A, Lehuen A.. MAIT cells, guardians of skin and mucosa?. Mucosal Immunol 2021, 14, 803–14. doi: 10.1038/s41385-021-00391-w [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89. D’Souza C, Pediongco T, Wang H, Scheerlinck JY, Kostenko L, Esterbauer R, et al. Mucosal-associated invariant T cells augment immunopathology and gastritis in chronic helicobacter pylori infection. J Immunol 2018, 200, 1901–16. doi: 10.4049/jimmunol.1701512 [DOI] [PubMed] [Google Scholar]
- 90. Ussher JE, van Wilgenburg B, Hannaway RF, Ruustal K, Phalora P, Kurioka A, et al. TLR signaling in human antigen-presenting cells regulates MR1-dependent activation of MAIT cells. Eur J Immunol 2016, 46, 1600–14. doi: 10.1002/eji.201545969 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91. Croxford JL, Miyake S, Huang YY, Shimamura M, Yamamura T.. Invariant V(alpha)19i T cells regulate autoimmune inflammation. Nat Immunol 2006, 7, 987–94. doi: 10.1038/ni1370 [DOI] [PubMed] [Google Scholar]
- 92. Abrahamsson SV, Angelini DF, Dubinsky AN, Morel E, Oh U, Jones JL, et al. Non-myeloablative autologous haematopoietic stem cell transplantation expands regulatory cells and depletes IL-17 producing mucosal-associated invariant T cells in multiple sclerosis. Brain 2013, 136, 2888–903. doi: 10.1093/brain/awt182 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93. Sakai S, Kauffman KD, Oh S, Nelson CE, Barry CE 3rd, Barber DL.. MAIT cell-directed therapy of Mycobacterium tuberculosis infection. Mucosal Immunol 2021, 14, 199–208. doi: 10.1038/s41385-020-0332-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94. Dey RJ, Dey B, Harriff M, Canfield ET, Lewinsohn DM, Bishai WR.. Augmentation of the riboflavin-biosynthetic pathway enhances mucosa-associated invariant T (MAIT) cell activation and diminishes Mycobacterium tuberculosis virulence. mBio 2022, 13, e0386521. doi: 10.1128/mbio.03865-21 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95. Gold MC, Cerri S, Smyk-Pearson S, Cansler ME, Vogt TM, Delepine J, et al. Human mucosal associated invariant T cells detect bacterially infected cells. PLoS Biol 2010, 8, e1000407. doi: 10.1371/journal.pbio.1000407 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96. Le Bourhis L, Dusseaux M, Bohineust A, Bessoles S, Martin E, Premel V, et al. MAIT cells detect and efficiently lyse bacterially-infected epithelial cells. PLoS Pathog 2013, 9, e1003681. doi: 10.1371/journal.ppat.1003681 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97. Lett MJ, Mehta H, Keogh A, Jaeger T, Jacquet M, Powell K, et al. Stimulatory MAIT cell antigens reach the circulation and are efficiently metabolised and presented by human liver cells. Gut 2022, 71, 2526–38. doi: 10.1136/gutjnl-2021-324478 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98. Lepore M, Kalinichenko A, Calogero S, Kumar P, Paleja B, Schmaler M, et al. Functionally diverse human T cells recognize non-microbial antigens presented by MR1. Elife 2017, 6, e24476. doi: 10.7554/eLife.24476 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99. Keller AN, Eckle SB, Xu W, Liu L, Hughes VA, Mak JY, et al. Drugs and drug-like molecules can modulate the function of mucosal-associated invariant T cells. Nat Immunol 2017, 18, 402–11. doi: 10.1038/ni.3679 [DOI] [PubMed] [Google Scholar]
- 100. Sattler A, Dang-Heine C, Reinke P, Babel N.. IL-15 dependent induction of IL-18 secretion as a feedback mechanism controlling human MAIT-cell effector functions. Eur J Immunol 2015, 45, 2286–98. doi: 10.1002/eji.201445313 [DOI] [PubMed] [Google Scholar]
- 101. Jo J, Tan AT, Ussher JE, Sandalova E, Tang XZ, Tan-Garcia A, et al. Toll-like receptor 8 agonist and bacteria trigger potent activation of innate immune cells in human liver. PLoS Pathog 2014, 10, e1004210. doi: 10.1371/journal.ppat.1004210 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102. Wang H, Kjer-Nielsen L, Shi M, D’Souza C, Pediongco TJ, Cao H, et al. IL-23 costimulates antigen-specific MAIT cell activation and enables vaccination against bacterial infection. Sci Immunol 2019, 4, eaaw0402. doi: 10.1126/sciimmunol.aaw0402. [DOI] [PubMed] [Google Scholar]
- 103. Ming S, Zhang M, Liang Z, Li C, He J, Chen P, et al. OX40L/OX40 signal promotes IL-9 production by mucosal MAIT cells during Helicobacter pylori infection. Front Immunol 2021, 12, 626017. doi: 10.3389/fimmu.2021.626017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104. Xiao X, Cai J.. Mucosal-associated invariant t cells: new insights into antigen recognition and activation. Front Immunol 2017, 8, 1540. doi: 10.3389/fimmu.2017.01540 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105. Chandra S, Kronenberg M.. Activation and function of iNKT and MAIT cells. Adv Immunol 2015, 127, 145–201. doi: 10.1016/bs.ai.2015.03.003 [DOI] [PubMed] [Google Scholar]
- 106. Haeryfar SMM, Shaler CR, Rudak PT.. Mucosa-associated invariant T cells in malignancies: a faithful friend or formidable foe?. Cancer Immunol Immunother 2018, 67, 1885–96. doi: 10.1007/s00262-018-2132-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107. Kurioka A, van Wilgenburg B, Javan RR, Hoyle R, van Tonder AJ, Harrold CL, et al. Diverse Streptococcus pneumoniae strains drive a mucosal-associated invariant T-cell response through major histocompatibility complex class I-related molecule-dependent and cytokine-driven pathways. J Infect Dis 2018, 217, 988–99. doi: 10.1093/infdis/jix647 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108. Georgel P, Radosavljevic M, Macquin C, Bahram S.. The non-conventional MHC class I MR1 molecule controls infection by Klebsiella pneumoniae in mice. Mol Immunol 2011, 48, 769–75. doi: 10.1016/j.molimm.2010.12.002 [DOI] [PubMed] [Google Scholar]
- 109. Zhao Z, Wang H, Shi M, Zhu T, Pediongco T, Lim XY, et al. Francisella tularensis induces Th1 like MAIT cells conferring protection against systemic and local infection. Nat Commun 2021, 12, 4355. doi: 10.1038/s41467-021-24570-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110. Dusseaux M, Martin E, Serriari N, Péguillet I, Premel V, Louis D, et al. Human MAIT cells are xenobiotic-resistant, tissue-targeted, CD161hi IL-17-secreting T cells. Blood 2011, 117, 1250–9. doi: 10.1182/blood-2010-08-303339 [DOI] [PubMed] [Google Scholar]
- 111. Godfrey DI, Koay HF, McCluskey J, Gherardin NA.. The biology and functional importance of MAIT cells. Nat Immunol 2019, 20, 1110–28. doi: 10.1038/s41590-019-0444-8 [DOI] [PubMed] [Google Scholar]
- 112. Boulouis C, Sia WR, Gulam MY, Teo JQM, Png YT, Phan TK, et al. Human MAIT cell cytolytic effector proteins synergize to overcome carbapenem resistance in Escherichia coli. PLoS Biol 2020, 18, e3000644. doi: 10.1371/journal.pbio.3000644 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113. Boulouis C, Gorin JB, Dias J, Bergman P, Leeansyah E, Sandberg JK.. Opsonization-enhanced antigen presentation by MR1 activates rapid polyfunctional MAIT cell responses acting as an effector arm of humoral antibacterial immunity. J Immunol 2020, 205, 67–77. doi: 10.4049/jimmunol.2000003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114. Salou M, Lantz O.. A TCR-dependent tissue repair potential of MAIT cells. Trends Immunol 2019, 40, 975–7. doi: 10.1016/j.it.2019.09.001 [DOI] [PubMed] [Google Scholar]
- 115. du Halgouet A, Darbois A, Alkobtawi M, Mestdagh M, Alphonse A, Premel V, et al. Role of MR1-driven signals and amphiregulin on the recruitment and repair function of MAIT cells during skin wound healing. Immunity 2023, 56, 78–92.e6. doi: 10.1016/j.immuni.2022.12.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116. Ribot JC, Lopes N, Silva-Santos B.. γδ T cells in tissue physiology and surveillance. Nat Rev Immunol 2021, 21, 221–32. doi: 10.1038/s41577-020-00452-4 [DOI] [PubMed] [Google Scholar]
- 117. Jameson J, Ugarte K, Chen N, Yachi P, Fuchs E, Boismenu R, et al. A role for skin gammadelta T cells in wound repair. Science 2002, 296, 747–9. doi: 10.1126/science.1069639 [DOI] [PubMed] [Google Scholar]
- 118. Linehan JL, Harrison OJ, Han SJ, Byrd AL, Vujkovic-Cvijin I, Villarino AV, et al. Non-classical immunity controls microbiota impact on skin immunity and tissue repair. Cell 2018, 172, 784–796.e18. doi: 10.1016/j.cell.2017.12.033 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119. Downey AM, Kapłonek P, Seeberger PH.. MAIT cells as attractive vaccine targets. FEBS Lett 2019, 593, 1627–40. doi: 10.1002/1873-3468.13488 [DOI] [PubMed] [Google Scholar]
- 120. Jensen O, Trivedi S, Li K, Aubé J, Hale JS, Ryan ET, et al. Use of a MAIT-activating ligand, 5-OP-RU, as a mucosal adjuvant in a murine model of Vibrio cholerae O1 vaccination. Pathog Immun 2022, 7, 122–44. doi: 10.20411/pai.v7i1.525 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121. Bennett MS, Trivedi S, Iyer AS, Hale JS, Leung DT.. Human mucosal-associated invariant T (MAIT) cells possess capacity for B cell help. J Leukoc Biol 2017, 102, 1261–9. doi: 10.1189/jlb.4A0317-116R [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122. Provine NM, Amini A, Garner LC, Spencer AJ, Dold C, Hutchings C, et al. MAIT cell activation augments adenovirus vector vaccine immunogenicity. Science 2021, 371, 521–6. doi: 10.1126/science.aax8819 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123. Salio M, Gasser O, Gonzalez-Lopez C, Martens A, Veerapen N, Gileadi U, et al. Activation of human mucosal-associated invariant T cells induces CD40L-dependent maturation of monocyte-derived and primary dendritic cells. J Immunol 2017, 199, 2631–8. doi: 10.4049/jimmunol.1700615 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124. Schneider M, Hannaway RF, Lamichhane R, de la Harpe SM, Tyndall JDA, Vernall AJ, et al. Neutrophils suppress mucosal-associated invariant T cells in humans. Eur J Immunol 2020, 50, 643–55. doi: 10.1002/eji.201948394 [DOI] [PubMed] [Google Scholar]
- 125. Yamamoto T, Kita M, Ohno T, Iwakura Y, Sekikawa K, Imanishi J.. Role of tumor necrosis factor-alpha and interferon-gamma in Helicobacter pylori infection. Microbiol Immunol 2004, 48, 647–54. doi: 10.1111/j.1348-0421.2004.tb03474.x [DOI] [PubMed] [Google Scholar]
- 126. Law BMP, Wilkinson R, Wang X, Kildey K, Giuliani K, Beagley KW, et al. Human tissue-resident mucosal-associated invariant T (MAIT) cells in renal fibrosis and CKD. J Am Soc Nephrol 2019, 30, 1322–35. doi: 10.1681/ASN.2018101064 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127. Riva A, Patel V, Kurioka A, Jeffery HC, Wright G, Tarff S, et al. Mucosa-associated invariant T cells link intestinal immunity with antibacterial immune defects in alcoholic liver disease. Gut 2018, 67, 918–30. doi: 10.1136/gutjnl-2017-314458 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128. Jeffery HC, van Wilgenburg B, Kurioka A, Parekh K, Stirling K, Roberts S, et al. Biliary epithelium and liver B cells exposed to bacteria activate intrahepatic MAIT cells through MR1. J Hepatol 2016, 64, 1118–27. doi: 10.1016/j.jhep.2015.12.017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129. Cesaro C, Tiso A, Del Prete A, Cariello R, Tuccillo C, Cotticelli G, et al. Gut microbiota and probiotics in chronic liver diseases. Dig Liver Dis 2011, 43, 431–8. doi: 10.1016/j.dld.2010.10.015 [DOI] [PubMed] [Google Scholar]
- 130. Almeida J, Galhenage S, Yu J, Kurtovic J, Riordan SM.. Gut flora and bacterial translocation in chronic liver disease. World J Gastroenterol 2006, 12, 1493–502. doi: 10.3748/wjg.v12.i10.1493 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131. McWilliam HEG, Eckle SBG, Theodossis A, Liu L, Chen Z, Wubben JM, et al. The intracellular pathway for the presentation of vitamin B-related antigens by the antigen-presenting molecule MR1. Nat Immunol 2016, 17, 531–7. doi: 10.1038/ni.3416 [DOI] [PubMed] [Google Scholar]
- 132. Nejman D, Livyatan I, Fuks G, Gavert N, Zwang Y, Geller LT, et al. The human tumor microbiome is composed of tumor type–specific intracellular bacteria. Science 2020, 368, 973–80. doi: 10.1126/science.aay9189 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133. Li S, Simoni Y, Becht E, Loh CY, Li N, Lachance D, et al. Human tumor-infiltrating MAIT cells display hallmarks of bacterial antigen recognition in colorectal cancer. Cell Rep Med 2020, 1, 100039. doi: 10.1016/j.xcrm.2020.100039 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134. Blosse A, Lehours P, Wilson KT, Gobert AP.. Helicobacter: inflammation, immunology, and vaccines. Helicobacter 2018, 23, e12517. doi: 10.1111/hel.12517 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135. Ye X, Li Y, Fang B, Yuan Y, Feng D, Chen H, et al. Type 17 mucosal-associated invariant T cells contribute to neutrophilic inflammation in patients with nasal polyps. J Allergy Clin Immunol 2023, S0091-6749(23)00861-8. doi: 10.1016/j.jaci.2023.06.021 [DOI] [PubMed] [Google Scholar]
- 136. Cooper AM. IL-17 and anti-bacterial immunity: protection versus tissue damage. Eur J Immunol 2009, 39, 649–52. doi: 10.1002/eji.200839090 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137. Duruel O, Berker E, Özşin-Özler C, Gharibzadeh-Hızal M, Gürpınar O, Eryılmaz-Polat S, et al. Levels of pro- and anti-inflammatory cytokines in cystic fibrosis patients with or without gingivitis. Cytokine 2020, 127, 154987. doi: 10.1016/j.cyto.2020.154987 [DOI] [PubMed] [Google Scholar]
- 138. Lindén A, Adachi M.. Neutrophilic airway inflammation and IL-17. Allergy 2002, 57, 769–75. doi: 10.1034/j.1398-9995.2002.02164.x [DOI] [PubMed] [Google Scholar]
- 139. Kulig P, Burkhard S, Mikita-Geoffroy J, Croxford AL, Hövelmeyer N, Gyülvészi G, et al. IL17A-mediated endothelial breach promotes metastasis formation. Cancer Immunol Res 2016, 4, 26–32. doi: 10.1158/2326-6066.CIR-15-0154 [DOI] [PubMed] [Google Scholar]
- 140. Loman BR, Russart KLG, Grant CV, Lynch AJ, Bailey MT, Pyter LM.. Mammary tumors alter the fecal bacteriome and permit enteric bacterial translocation. BMC Cancer 2022, 22, 245. doi: 10.1186/s12885-022-09274-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141. Sakai S, Lora NE, Kauffman KD, Dorosky DE, Oh S, Namasivayam S, et al. Functional inactivation of pulmonary MAIT cells following 5-OP-RU treatment of non-human primates. Mucosal Immunol 2021, 14, 1055–66. doi: 10.1038/s41385-021-00425-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142. Yu H, Yang A, Liu L, Mak JYW, Fairlie DP, Cowley S.. CXCL16 stimulates antigen-induced MAIT cell accumulation but trafficking during lung infection is CXCR6-independent. Front Immunol 2020, 11, 1773. doi: 10.3389/fimmu.2020.01773 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143. De Biasi S, Gibellini L, Lo Tartaro D, Puccio S, Rabacchi C, Mazza EMC, et al. Circulating mucosal-associated invariant T cells identify patients responding to anti-PD-1 therapy. Nat Commun 2021, 12, 1669. doi: 10.1038/s41467-021-21928-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144. Jiang J, Wang X, An H, Yang B, Cao Z, Liu Y, et al. Mucosal-associated invariant T-cell function is modulated by programmed death-1 signaling in patients with active tuberculosis. Am J Respir Crit Care Med 2014, 190, 329–39. doi: 10.1164/rccm.201401-0106OC [DOI] [PubMed] [Google Scholar]
- 145. Saeidi A, Tien Tien VL, Al-Batran R, Al-Darraji HA, Tan HY, Yong YK, et al. Attrition of TCR Vα7.2+ CD161++ MAIT cells in HIV-tuberculosis co-infection is associated with elevated levels of PD-1 expression. PLoS One 2015, 10, e0124659. doi: 10.1371/journal.pone.0124659 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146. Yong YK, Saeidi A, Tan HY, Rosmawati M, Enström PF, Batran RA, et al. Hyper-expression of PD-1 is associated with the levels of exhausted and dysfunctional phenotypes of circulating CD161(++)TCR iVα7.2(+) mucosal-associated invariant T cells in chronic hepatitis B virus infection. Front Immunol 2018, 9, 472. doi: 10.3389/fimmu.2018.00472 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147. Xu-Monette ZY, Zhou J, Young KH.. PD-1 expression and clinical PD-1 blockade in B-cell lymphomas. Blood 2018, 131, 68–83. doi: 10.1182/blood-2017-07-740993 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148. Patel O, Kjer-Nielsen L, Le Nours J, Eckle SB, Birkinshaw R, Beddoe T, et al. Recognition of vitamin B metabolites by mucosal-associated invariant T cells. Nat Commun 2013, 4, 2142. doi: 10.1038/ncomms3142 [DOI] [PubMed] [Google Scholar]
- 149. Eckle SB, Birkinshaw RW, Kostenko L, Corbett AJ, McWilliam HE, Reantragoon R, et al. A molecular basis underpinning the T cell receptor heterogeneity of mucosal-associated invariant T cells. J Exp Med 2014, 211, 1585–600. doi: 10.1084/jem.20140484 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150. Yasutomi Y, Chiba A, Haga K, Murayama G, Makiyama A, Kuga T, et al. Activated mucosal-associated invariant T cells have a pathogenic role in a murine model of inflammatory bowel disease. Cell Mol Gastroenterol Hepatol 2022, 13, 81–93. doi: 10.1016/j.jcmgh.2021.08.018 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


