Abstract
Activity-regulated cytoskeleton associated protein (Arc1), which is required for synaptic plasticity and metabolism in Drosophila , self-assembles into capsid-like structures that transport mRNAs in extracellular vesicles. In addition to expression in the brain and nervous system, Arc1 is expressed in the male accessory glands, an endothelial tissue that produces male seminal proteins and exosomes that impact male fertility. We thus hypothesized that Arc1 might impact male fertility. We measured the fertility, mating latency, mating duration, and sperm competition performance of Arc1 males relative to controls and found no evidence that Arc1 is required for any of these measures of male fertility.
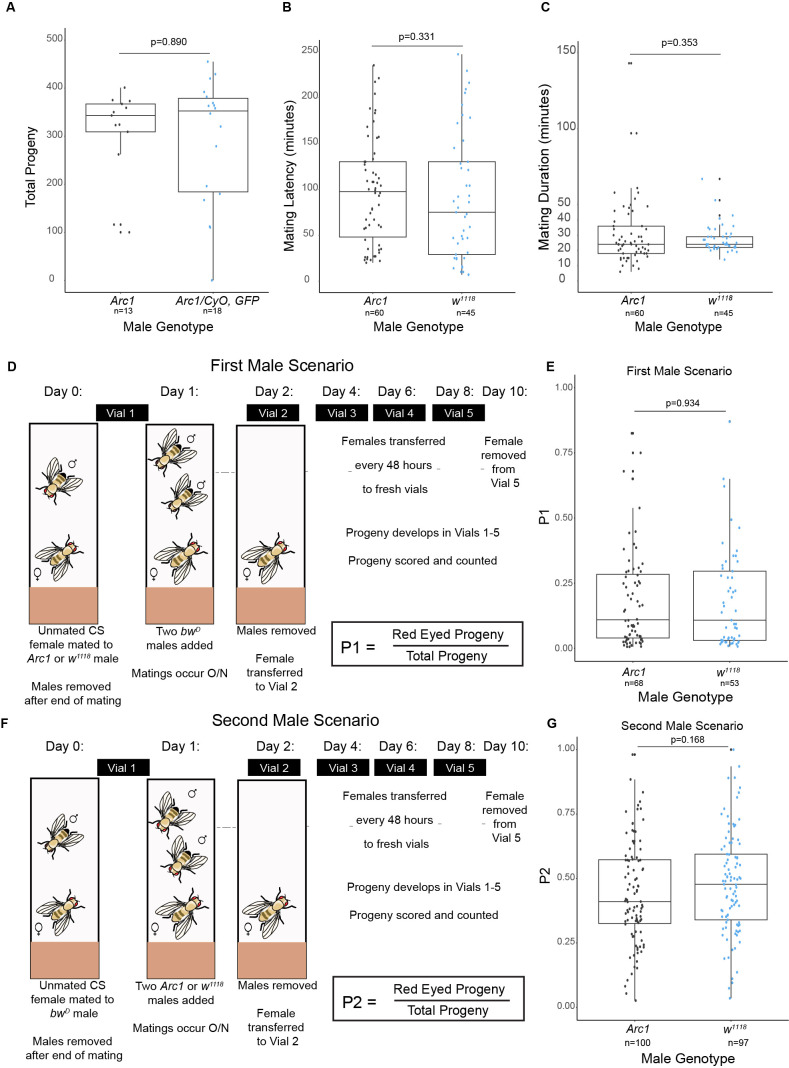
Figure 1. Arc1 is not required for male fertility, copulation, or sperm competition success.
A) Total progeny produced by homozygous Arc1 mutants (n=13) and heterozygous Arc1/CyO, GFP control males (n=18). Wilcoxon rank sum test: p =0.890). B) Mating latency of Arc1 mutants (n=60) and w 1118 controls (n=45). Welch’s t-test: p =0.311 C) Mating duration of Arc1 mutants (n=60) and w 1118 controls (n=45). Welch’s t-test: p =0.353. D) First male experimental set up, where Arc1 mutants or w 1118 control males were first to mate with wild-type females, followed by bw D males. E) Paternal share (P1) of Arc1 mutants (n=68) and w 1118 controls (n=53) in first male scenario. Two way t-test: p =0.934 F) Second male experimental set up, where bw D males were first to mate with wild-type females, followed by Arc1 mutants or w 1118 control males. G) Paternal share (P2) of Arc1 mutants (n=100) and w 1118 controls (n=97) in second male scenario. Two way t-test: p =0.168.
Description
Activity-regulated cytoskeleton associated protein (ARC) is an immediate-early gene that responds to neuronal activity (reviewed in Shepherd & Bear, 2011) . Mammalian ARC is required for synaptic plasticity and long term memory, and mutations in human Arc are associated with neuronal disorders like schizophrenia, Alzheimer’s, and autism spectrum disorders (reviewed in Shepherd & Bear, 2011) . Both mammalian ARC and Drosophila Arc1 contain retroviral group-specific, antigen-like amino acid sequences that are thought to derive from Ty3 retrotransposons (Cottee et al., 2020) . Using a Gag-like protein, both mammalian ARC and Drosophila Arc1 form capsids containing their mRNA that are packaged inside extracellular vesicles which can be secreted from neurons (Ashley et al., 2018; Pastuzyn et al., 2018) . In Drosophila melanogaster , Arc1 is associated with synapse maturation and plasticity (Ashley et al., 2018) . Additionally, Arc1 mutants have altered metabolism, high levels of fat storage, and are resistant to starvation (Keith et al., 2021; Mattaliano et al., 2007; Mosher et al., 2015) . Furthermore, Keith et al. (2021) showed that Arc1 interacts with the microbiome to regulate growth and metabolic homeostasis.
Although mammalian Arc and Drosophila Arc1 are primarily expressed in the brain and nervous system, both also show reproductive tract expression. Mammalian Arc is expressed in testes (Shepherd & Bear, 2011) and Arc1 is expressed in the Drosophila male accessory gland (Leader et al., 2018) . The male accessory gland is an endothelial secretory tissue that produces seminal proteins and exosomes that are transferred to females during mating (reviewed in Avila et al., 2011; Wilson et al., 2017). These male accessory gland molecules then regulate female post-mating behaviors such as refractoriness to remating and sustained egg production (reviewed in Avila et al., 2011; Wilson et al., 2017). However, it is not known whether Arc plays a role in male reproductive success in either mammals or insects.
We found that homozygous mutant w 1118 ; Arc1 E8 ( Arc1 ) males sire a normal number of progeny, indicating that Arc1 is not required for male fertility ( Figure 1A : Wilcoxon Rank Sum Test, p =0.890). However, multiple other traits impact male reproductive success, including the relative success of a male in competition with other males. Because female Drosophila melanogaster can mate with multiple mates and store sperm for days to weeks, male ejaculates can compete for reproductive success within the female, as reflected in paternal progeny proportion (Bloch Qazi et al., 2003; Giardina et al., 2017; Neubaum & Wolfner, 1999) . Seminal fluid components derived from the male accessory gland, as well as sperm length, number, and speed, are known to influence paternity proportion (Castillo & Moyle, 2014; Chow et al., 2013; Clark et al., 1995; Clark & Begun, 1998; Fiumera et al., 2005, 2007; Fricke et al., 2009; Zhang et al., 2013) . Furthermore, in response to competition with a rival, male D. melanogaster can decrease mating latency and increase mating duration, which can ultimately lead to an increase in paternal progeny proportion (Bretman et al., 2009) . Changes in mating duration in competitive environments are linked to memory processing through activity in the mushroom body (Rouse et al., 2018) . Given the role of Arc1 in synapse plasticity (Ashley et al., 2018) and its expression in male accessory gland (Leader et al., 2018) , we hypothesized that dArc1 could affect mating duration and ultimately sperm competition success.
To test our hypothesis, we observed matings between wild-type Canton S (CS) females and Arc1 mutant or w 1118 control males, and measured both mating latency and mating duration. We found no difference in mating latency ( Figure 1B : Welch’s t-test, p= 0.331) or mating duration ( Figure 1C : Welch’s t-test, p= 0.353) between Arc1 mutants and w 1118 controls. We next assayed the sperm competition success of Arc1 mutants in two different experimental set-ups, competing Arc1 or w 1118 control males against males with a dominant allele of the eye color gene, brown ( bw D ). In the first male scenario, Arc1 or w 1118 control males were mated to a female first, followed by the second mate: bw D males ( Figure 1D ). In the second male scenario, females were first mated to a bw D male, followed by either an Arc1 or a w 1118 control male ( Figure 1F ). In both competitive experimental set-ups, the paternity of each progeny could be determined by eye color: all progeny of bw D males and the CS females have brown eyes while all progeny of Arc1 or w 1118 males and the CS female have wildtype eyes. For the first male scenario, we calculated the paternity proportion (P1) of the first male ( Arc1 or w 1118 ) as the total number of first male progeny divided by the total number of progeny. For the second male scenario, we calculated the paternity proportion (P2) of the second male ( Arc1 or w 1118 ) as the total number of second male progeny divided by the total number of progeny. If Arc1 influences male sperm competition success, we would expect that Arc1 mutant males will yield lower proportions of progeny relative to controls. Conversely, if Arc1 is not required for male sperm competitive success, we would expect dArc1 mutant males to produce similar proportions of progeny as control males.
We found no difference in P1 between Arc1 mutants and w 1118 controls ( Figure 1E, two sample t -test, p =0.934). Similarly, we found no difference in P2 between Arc1 mutants and w 1118 controls ( Figure 1G, two sample t -test, p =0.168). Thus, despite the expression of Arc1 in both the nervous system and the male reproductive tract, we found no evidence of fertility defects, or impaired mating latency, duration, or sperm competition success in Arc1 mutant males.
These negative results indicate that Arc1 is not required for Drosophila male reproductive success. However, the role of Drosophila Arc1 and mammalian ARC in male reproductive tissues remains to be elucidated. Future studies to define the localization of Arc1 in the male accessory gland or comparative transcriptomics and proteomics on Arc1 mutant versus control accessory glands could help identify a functional role for Arc1 in male reproductive health.
Methods
Fly Husbandry and Genotypes
All flies were raised on cornmeal-sucrose media (weight by volume in 1 L of H 2 O: 0.7% agar, 6% Brewer’s yeast, 6% cornmeal, and 4% sucrose with 26.5 mL of 100g Tegosept in 95% ethanol and 12 mL mixture of 0.04% phosphoric acid and 0.4% propionic acid to inhibit microbial growth). All flies were kept on a 12-hour light-dark cycle at room temperature (22-25 o C). Arc1 mutants were a gift from Dr. Travis Thomson and are w 1118 ; Arc1 E8 or the balanced control, w 1118 ; Arc1 E8 /CyO, GFP (Ashley et al., 2018) . w 1118 males were used as genetic background controls. The males with a dominant allele of brown (designated bw D ) are cn bw D . All females were wild-type Canton S.
Male Fertility Test
We tested the fertility of w 1118 ; Arc1 E8 (Arc1) mutant males compared to heterozygous control males, w 1118 ; Arc1 E8 /CyO, GFP ( Arc1/CyO, GFP) . Single wild-type CS females (3-5 days old) were aspirated into a fresh food vial treated with dry yeast the night before the matings occurred. In the morning, single Arc1 or Arc1/CyO, GFP males were aspirated into a vial containing a single female. Matings were observed and the start and end times of copulation were recorded. After mating, males were removed from the vial. Females were transferred to new food vials every 24 hours for 4 days, thereby creating four total vials per female. Progeny were allowed to develop to the adult stage, then counted. Total progeny is reported as the sum of progeny from all four vials per individual male. We compared the mean total progeny of Arc1 and Arc1/CyO, GFP males by a Wilcoxon rank sum test.
Mating Duration and Latency
Mating latency is the length of time between introduction of the first male mate and initiation of copulation. Mating duration is the length of time from the initiation of copulation to the end of copulation. We used Welch’s t-test to compare both mean mating latency and mean mating duration between Arc1 mutants and w 1118 controls.
Sperm Competition Experimental Design
The sperm competition assay was based on (Chen et al., 2019) and (Chen et al., 2022) . The success of Arc1 mutants was assayed in two different sperm competition experimental set-ups where Arc1 males and w 1118 controls were either the first or second male to mate with a single female. In the first male scenario, wild-type Canton S females were set up in single-pair matings with either Arc1 or w 1118 males on day 0 in vial 1. Copulations were observed and males were removed after mating ended. In the evening of day 1, two bw D males were added to each vial and matings were allowed to occur overnight. In the morning of day 2, the bw D males were removed and all females were transferred to vial 2. Each female was transferred every 48 hours for 8 days to a new vial for a total of 5 vials. Females were discarded on day 10 and all progeny were allowed to develop. Progeny were separated by eye color and counted. The paternity of the progeny was determined by eye color: progeny from Arc1 or w 1118 fathers had wild-type eyes, while progeny from bw D fathers had brown eyes. In the second-male scenario, the same procedure was performed except bw D males were first mated to Canton S females on day 0, while two Arc1 or w 1118 males were added in the evening of day 1.
For both sperm competition scenarios, the experiment was performed in two blocks. The experimenter counting progeny was blinded to the first or second male’s genotypic identity ( Arc1 or w 1118 ) by keeping the male identities in a key until progeny data were analyzed.
Paternal Success of First Male (P1) and Second Male (P2)
Females that did not survive the 10 days of the experiment or did not produce progeny from each of the two mates were discarded. In the first male scenario, paternal progeny proportion for the first male (P1) was calculated as the number of first male progeny divided by the total number of progeny. In the second male scenario, paternal progeny proportion for the second male (P2) was calculated as the number of second male progeny divided by the total number of progeny. Although both mating events took place in vial 1, there was more opportunity for fertilized eggs to be laid by the first male. Therefore, we discarded this vial for counts of P1 and P2. We compared mean P1 or P2 for dArc1 mutants and w 1118 controls using a two sample t-test.
Data Analysis and Visualization
All data was analyzed using R Studio Version 2023.06.0+421. All graphs were created using the package ggplot2. Experimental set-up figures were created and arranged with graphs using Adobe Illustrator. Drosophila cartoons are licensed under Creative Commons 3.0.
Acknowledgments
Acknowledgments
We thank Dr. Scott Keith for inspiring this investigation and Dr. Travis Thomson for sharing the Arc1 mutant lines. We also thank Dr. Dawn Chen and Dr. Nora Brown for assistance with experimental design and data analysis.
References
- Ashley James, Cordy Benjamin, Lucia Diandra, Fradkin Lee G., Budnik Vivian, Thomson Travis. Retrovirus-like Gag Protein Arc1 Binds RNA and Traffics across Synaptic Boutons. Cell. 2018 Jan 1;172(1-2):262–274.e11. doi: 10.1016/j.cell.2017.12.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Avila Frank W., Sirot Laura K., LaFlamme Brooke A., Rubinstein C. Dustin, Wolfner Mariana F. Insect Seminal Fluid Proteins: Identification and Function. Annual Review of Entomology. 2011 Jan 7;56(1):21–40. doi: 10.1146/annurev-ento-120709-144823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bloch Qazi Margaret C, Heifetz Yael, Wolfner Mariana F. The developments between gametogenesis and fertilization: ovulation and female sperm storage in drosophila melanogaster. Developmental Biology. 2003 Apr 1;256(2):195–211. doi: 10.1016/s0012-1606(02)00125-2. [DOI] [PubMed] [Google Scholar]
- Bretman Amanda, Fricke Claudia, Chapman Tracey. Plastic responses of male Drosophila melanogaster to the level of sperm competition increase male reproductive fitness . Proceedings of the Royal Society B: Biological Sciences. 2009 Feb 25;276(1662):1705–1711. doi: 10.1098/rspb.2008.1878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Castillo Dean M., Moyle Leonie C. Intraspecific sperm competition genes enforce post-mating species barriers in Drosophila . Proceedings of the Royal Society B: Biological Sciences. 2014 Dec 22;281(1797):20142050–20142050. doi: 10.1098/rspb.2014.2050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen Dawn S, Clark Andrew G, Wolfner Mariana F. Octopaminergic/tyraminergic Tdc2 neurons regulate biased sperm usage in female Drosophila melanogaster . Genetics. 2022 Jul 9;221(4) doi: 10.1093/genetics/iyac096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen Dawn S, Delbare Sofie Y N, White Simone L, Sitnik Jessica, Chatterjee Martik, DoBell Elizabeth, Weiss Orli, Clark Andrew G, Wolfner Mariana F. Female Genetic Contributions to Sperm Competition in Drosophila melanogaster . Genetics. 2019 May 17;212(3):789–800. doi: 10.1534/genetics.119.302284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chow Clement Y, Wolfner Mariana F, Clark Andrew G. Large Neurological Component to Genetic Differences Underlying Biased Sperm Use in Drosophila . Genetics. 2013 Jan 1;193(1):177–185. doi: 10.1534/genetics.112.146357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clark A G, Aguadé M, Prout T, Harshman L G, Langley C H. Variation in sperm displacement and its association with accessory gland protein loci in Drosophila melanogaster. Genetics. 1995 Jan 1;139(1):189–201. doi: 10.1093/genetics/139.1.189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clark Andrew G, Begun David J. Female Genotypes Affect Sperm Displacement in Drosophila. Genetics. 1998 Jul 1;149(3):1487–1493. doi: 10.1093/genetics/149.3.1487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cottee Matthew A., Letham Suzanne C., Young George R., Stoye Jonathan P., Taylor Ian A. Structure of Drosophila melanogaster ARC1 reveals a repurposed molecule with characteristics of retroviral Gag . Science Advances. 2020 Jan 3;6(1) doi: 10.1126/sciadv.aay6354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fiumera Anthony C, Dumont Bethany L, Clark Andrew G. Sperm Competitive Ability in Drosophila melanogaster Associated With Variation in Male Reproductive Proteins. Genetics. 2005 Jan 1;169(1):243–257. doi: 10.1534/genetics.104.032870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fiumera Anthony C, Dumont Bethany L, Clark Andrew G. Associations Between Sperm Competition and Natural Variation in Male Reproductive Genes on the Third Chromosome of Drosophila melanogaster . Genetics. 2007 Jun 1;176(2):1245–1260. doi: 10.1534/genetics.106.064915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- FRICKE C., WIGBY S., HOBBS R., CHAPMAN T. The benefits of male ejaculate sex peptide transfer in Drosophila melanogaster . Journal of Evolutionary Biology. 2009 Jan 19;22(2):275–286. doi: 10.1111/j.1420-9101.2008.01638.x. [DOI] [PubMed] [Google Scholar]
- Giardina Thomas J., Clark Andrew G., Fiumera Anthony C. Estimating mating rates in wild Drosophila melanogaster females by decay rates of male reproductive proteins in their reproductive tracts . Molecular Ecology Resources. 2017 Mar 24;17(6):1202–1209. doi: 10.1111/1755-0998.12661. [DOI] [PubMed] [Google Scholar]
- Keith Scott A., Bishop Cassandra, Fallacaro Samantha, McCartney Brooke M. Arc1 and the microbiota together modulate growth and metabolic traits in Drosophila . Development. 2021 Jul 29;148(15) doi: 10.1242/dev.195222. [DOI] [PubMed] [Google Scholar]
- Leader David P, Krause Sue A, Pandit Aniruddha, Davies Shireen A, Dow Julian A T. FlyAtlas 2: a new version of the Drosophila melanogaster expression atlas with RNA-Seq, miRNA-Seq and sex-specific data. Nucleic Acids Research. 2017 Oct 24;46(D1):D809–D815. doi: 10.1093/nar/gkx976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mattaliano Mark D., Montana Enrico S., Parisky Katherine M., Littleton J. Troy, Griffith Leslie C. The Drosophila ARC homolog regulates behavioral responses to starvation. Molecular and Cellular Neuroscience. 2007 Oct 1;36(2):211–221. doi: 10.1016/j.mcn.2007.06.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mosher Jeremy, Zhang Wei, Blumhagen Rachel Z., D’Alessandro Angelo, Nemkov Travis, Hansen Kirk C., Hesselberth Jay R., Reis Tânia. Coordination between Drosophila Arc1 and a specific population of brain neurons regulates organismal fat. Developmental Biology. 2015 Sep 1;405(2):280–290. doi: 10.1016/j.ydbio.2015.07.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neubaum Deborah M., Wolfner Mariana F. 3 Wise, Winsome, or Weird? Mechanisms of Sperm Storage in Female Animals. Current Topics in Developmental Biology. 1998:67–97. doi: 10.1016/s0070-2153(08)60270-7. [DOI] [PubMed]
- Pastuzyn Elissa D., Day Cameron E., Kearns Rachel B., Kyrke-Smith Madeleine, Taibi Andrew V., McCormick John, Yoder Nathan, Belnap David M., Erlendsson Simon, Morado Dustin R., Briggs John A.G., Feschotte Cédric, Shepherd Jason D. The Neuronal Gene Arc Encodes a Repurposed Retrotransposon Gag Protein that Mediates Intercellular RNA Transfer. Cell. 2018 Jan 1;172(1-2):275–288.e18. doi: 10.1016/j.cell.2017.12.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rouse J., Watkinson K., Bretman A. Flexible memory controls sperm competition responses in male Drosophila melanogaster . Proceedings of the Royal Society B: Biological Sciences. 2018 May 30;285(1879):20180619–20180619. doi: 10.1098/rspb.2018.0619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shepherd Jason D, Bear Mark F. New views of Arc, a master regulator of synaptic plasticity. Nature Neuroscience. 2011 Jan 30;14(3):279–284. doi: 10.1038/nn.2708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilson, C., Leiblich, A., Goberdhan, D. C. I., Hamdy, F. 2017. Chapter Eleven - The Drosophila accessory gland as a model for prostate cancer and other pathologies. Current Topics in Developmental Biology. 121: 339. [DOI] [PMC free article] [PubMed]
- Zhang Rui, Clark Andrew G., Fiumera Anthony C. Natural genetic variation in male reproductive genes contributes to nontransitivity of sperm competitive ability in Drosophila melanogaster . Molecular Ecology. 2012 Nov 21;22(5):1400–1415. doi: 10.1111/mec.12113. [DOI] [PubMed] [Google Scholar]