FIGURE 2.
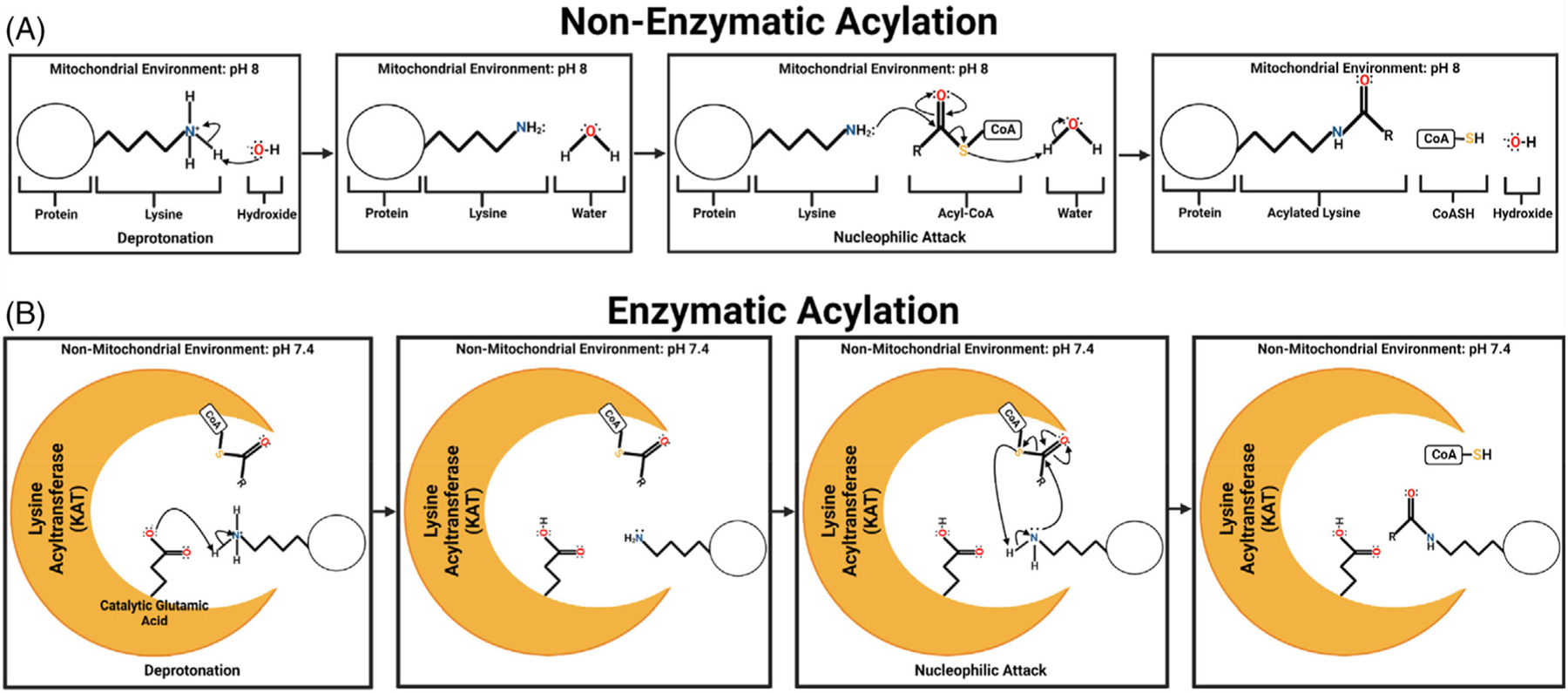
Nonenzymatic and Enzymatic Acylation. (A) Nonenzymatic acylation of lysine substrate. In the alkaline environment of the mitochondria, free hydroxide can serve as the proton acceptor to initiate nucleophilic attack of the Nε amino group of lysine on the reactive thioester of acyl-CoAs. The results are an acylated protein lysine residue, free CoASH, and reformation of hydroxide. (B) Enzymatic acylation of lysine substrate. Rather than free hydroxide, enzymatic acylation is initiated in the active pocket of a lysine acyltransferase or KAT through a catalytic amino acid (in this example it is glutamic acid) that initiates deprotonation. Deprotonation allows for nucleophilic attack of the Nε amino group of lysine on the reactive thioester of acyl-CoA which is held in proximity via binding to the KAT.
