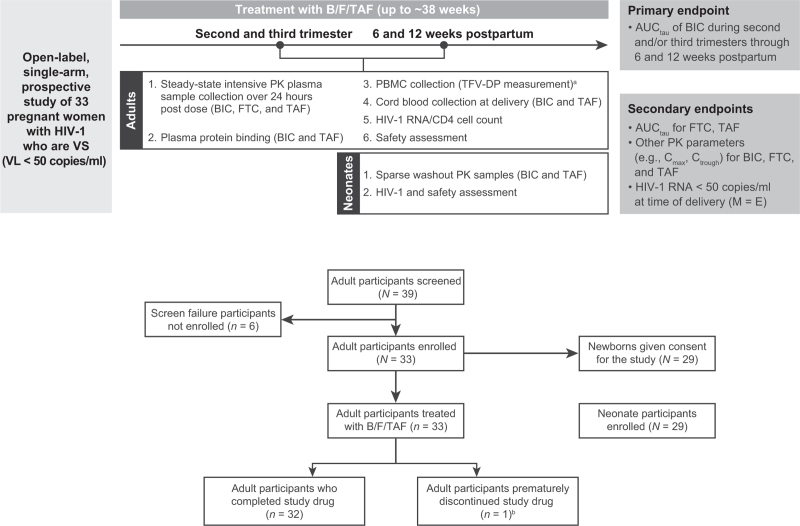
Fig. 1.
Study design and disposition of participants.
Neonates born to women participating in the study were followed from birth to 4–8 weeks of age if consent had been obtained from the parent/legal guardian. No neonatal participant in the study was treated with the study drug. aExploratory endpoint. bProtocol violation: M184V and other mutations at baseline, resulting in resistance to nucleoside/nucleotide reverse transcriptase inhibitor and nonnucleoside reverse transcriptase inhibitor treatment (n = 1). AUCtau, area under the plasma drug concentration–time curve over the dosing interval; B/F/TAF, bictegravir/emtricitabine/tenofovir alafenamide; BIC, bictegravir; Cmax, maximum observed plasma drug concentration; Ctrough, trough concentration; FTC, emtricitabine; HIV-1, HIV type 1; M = E, missing = excluded; PBMC, peripheral blood mononuclear cell; PK, pharmacokinetic; TAF, tenofovir alafenamide; TFV-DP, tenofovir diphosphate (active metabolite); VL, viral load; VS, virologically suppressed.