Fig. 2.
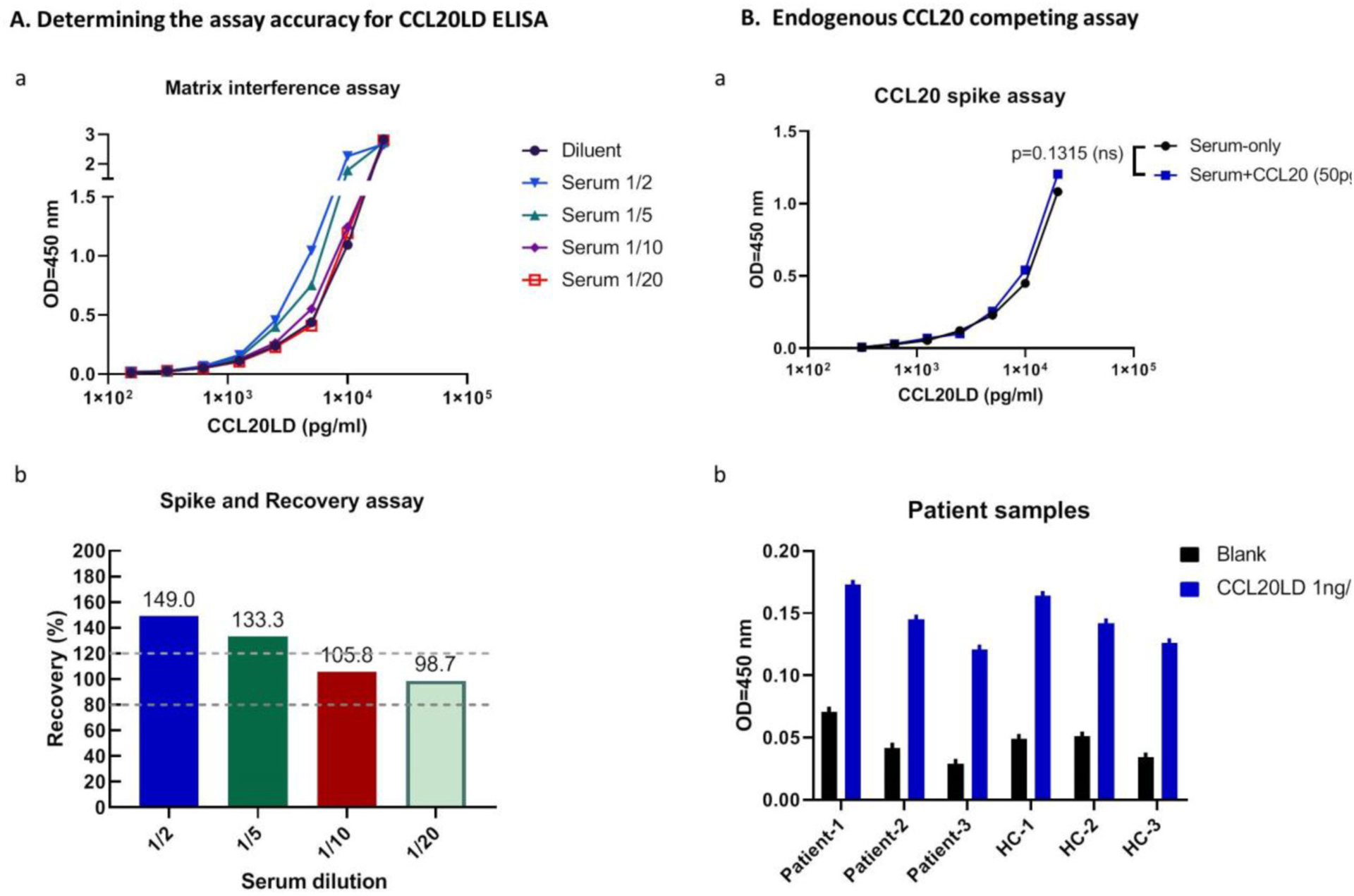
Assay accuracy and interference assessment for CCL20LD ELISA assay. A. (a) Comparison of OD values of CCL20LD in serum mixed at different ratios with standard diluent. Serial diluted CCL20LD in different matrix diluents, i.e., serum/diluent mixtures at the ratios of ½, 1/5, 1/10, or 1/20 were tested by CCL20LD ELISA to identify the Matrix interference. (b) Recovery of spiked CCL20LD in serum samples at different serum dilutions. Recovery rate is calculated for the percent of measured concentration in serum mixed samples to the expected concentration in diluent (percent values shown for the calculation with CCL20LD spiked at 5 ng/ml). Two dotted lines define the acceptable recovery rate between 80 and 120%. B. Probability evaluation of endogenous CCL20 interference from psoriatic specimen. (a) ELISA assay for CCL20LD in mouse serum spiked with and without CCL20 (50 pg/ml). OD values for serial diluted CCL20LD, ranging between 0 pg/ml and 12.5 ng/ml, showed no significant difference between the two groups (p = 0.1315). (b) CCL20LD ELISA assay was performed for plasma samples composed of both psoriasis patients and healthy controls (n = 3 in each group). OD values were shown for each sample spiked with, or without, CCL20LD protein.
