Abstract
Chemotherapy remains the most widely used cancer treatment modality. Nanotechnology provides exciting opportunities to improve these drugs, transforming decades-old generic treatments into precise new medicines. We illustrate the potential of recent advances in nanotechnology-enhanced therapy focusing on diffuse large B-cell lymphoma (DLBCL), the most common hematologic malignancy.
Keywords: Nanotechnology, Therapeutics, Drug Delivery, Lymphoma, Cancer, Precision Medicine
Emerging Landscape of Oncology Precision Medicines
Beginning in the mid-20th century, chemotherapy transformed cancer care, providing therapeutic options since used in millions of patients [1]. Now, technological advances in the new millennium are again transforming cancer medicine, built on genomics, proteomics, and detailed interrogation of tumor microenvironments that have revolutionized our picture of tumor biology [2–4]. Immunotherapies manipulating patients’ own immune systems to attack their tumors have generated high interest, with a host of therapies approved and under development [3]. Checkpoint inhibitors are monoclonal antibodies that reignite cytotoxic T-cell responses by blocking inhibitory signals tumors use to evade them [3]. Precision cellular immunotherapies – most notably chimeric antigen receptor-modified T (CAR-T) cells – are emerging, especially in selected hematologic malignancies [3]. These approaches are just some examples of precision medicines available in today’s cancer clinic, tailored to improve outcomes based on specific biomarkers. Targeted inhibitors of signaling kinases are others. These also have exploded in the new century, replacing old treatment paradigms almost completely in selected diseases like chronic leukemias and lung cancers driven by the anaplastic lymphoma kinase (ALK) oncogene. To this day, however, in most clinical scenarios, combination chemotherapy remains standard systemic therapy, especially in the frontline treatment setting and often decades after particular combinations first entered use [1]. The precision of new therapies comes at a price: Many tumors do not respond, and even for those that do, benefit to patients may be short-lived. Diffuse large B-cell lymphoma (DLBCL) is an illustrative example.
Diffuse large B-cell lymphoma
DLBCL is the most common hematologic cancer in the United States, an aggressive malignancy arising from mature B cells [5]. Readily identifiable oncogenic drivers include unique rearrangements between the constitutively expressed B-cell receptor (BCR) immunoglobulin heavy chain locus and the MYC, BCL2, and BCL6 oncogenes. This barely scratches the surface of DLBCL biologic, genomic, and clinical heterogeneity, however [5]. New integrated genomic and computational approaches have recently improved subclassification of DLBCL, comprising 5-7 major biologic subtypes [2, 4]. Regardless of subtype, however, chemotherapy is standard therapy in both the frontline and relapsed or refractory (rel/ref) settings. The combination CHOP (cyclophosphamide, doxorubicin, vincristine, prednisone), devised more than 40 years ago, became standard frontline therapy during the 80s and 90s [6]. Addition of the anti-CD20 antibody rituximab (R) in 2002 [7] has been the only real advance since that time, leaving R-CHOP as frontline standard therapy nearly two decades later (Fig. 1) [8]. Attempts to improve on R-CHOP’s ~60% cure rate have included addition of chemotherapy agents like etoposide and replacement of rituximab with next-generation anti-CD20 antibodies (Fig. 1). Dose-adjusted EPOCH-R (multi-day infusion dosing of the same drugs in R-CHOP plus etoposide), showed promising early-phase activity but didn’t improve outcomes compared to R-CHOP in a randomized phase III trial (Fig. 1). Intensified chemotherapy combinations (R-ACVBP, Fig. 1) did enhance efficacy compared to R-CHOP, but with significantly increased toxicity that prevented widespread implementation. For rel/ref disease, salvage chemoimmunotherapy combinations are standard but require stem-cell transplant consolidation for any meaningful chance of long-term disease-free survival [9]. Overall, availability of such approaches leads to cure for only about 1 in 10 rel/ref patients, taking into account those too frail or ill even to consider them [10]. The first decade and a half of the 21st century saw multiple targeted kinase/signaling inhibitors fail to find roles in the therapy of DLBCL. More recently, however, new options favoring targeted immunotherapies have emerged. These include the antibody drug conjugate polatuzumab vedotin against the BCR component CD79b [3]. CAR-T therapies directed at the pan-B marker CD19, however, have drawn the most attention, establishing proof of principle for reprogramming patients’ own immune cells ex vivo. Three “CAR-19” products are now FDA approved for DLBCL. Notwithstanding these exciting innovations, however, R-CHOP remains frontline standard across DLBCL, with several major attempts at improvement all failing. Though a ~60% frontline cure rate is better than for many malignancies, clearly there is room for improvement over R-CHOP for this common, deadly disease. In particular, a strategy we feel is prime for intensive investigation is use of nanotechnology guided by precise biomarker selection to achieve targeted delivery of existing chemotherapies (Fig. 2).
Figure 1: History of DLBCL Treatment.
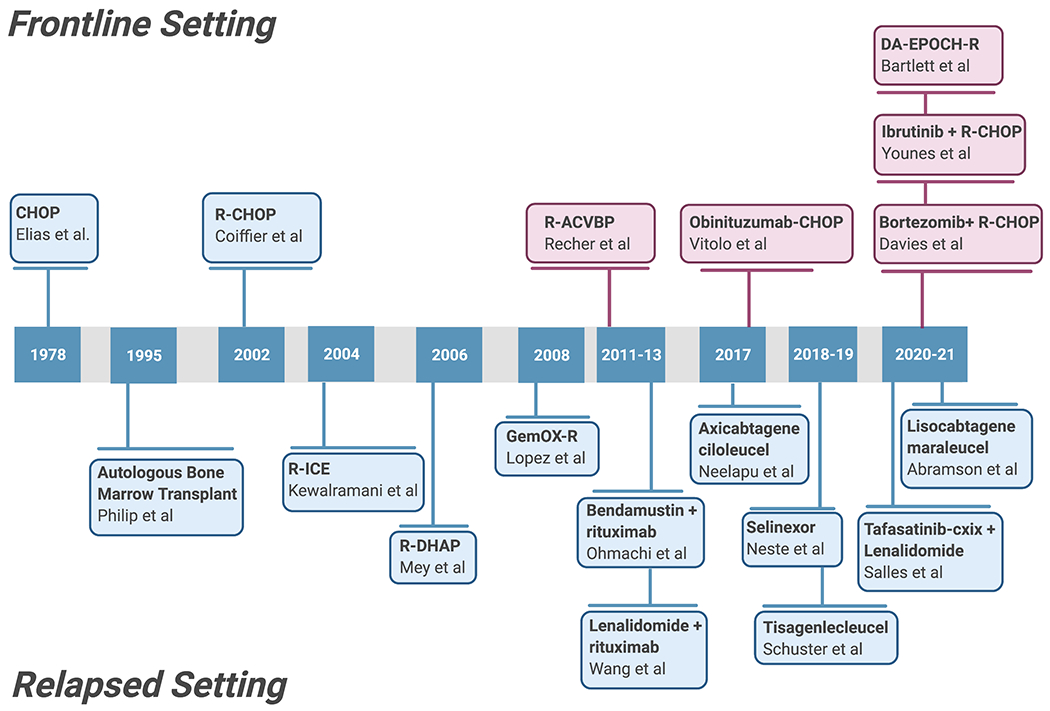
A timeline of new therapeutic regimens incorporated into DLBCL both in the frontline and relapsed patient settlings. Blue = successfully approved therapies; red = failed therapies.
Figure 2: Precision Nanomedicine.
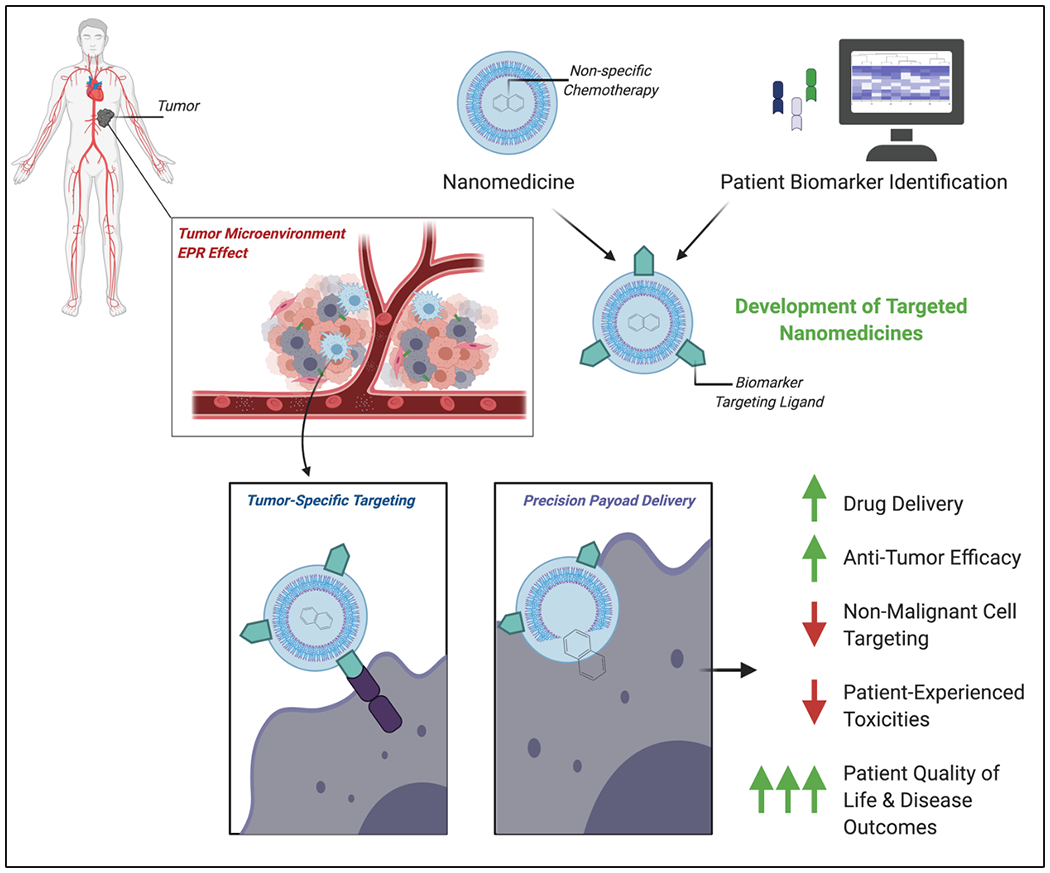
A graphic representation of combining nanotechnology with rationale patient biomarker identification for the development of targeted and optimized nanomedicines. Nano-optimized chemotherapy delivery capitalizes on the enhanced permeability and retention effect and enhanced precision of a tumor-specific targeting approach for the improvement of clinical outcomes.
Nanomedicine and Targeted Optimization
Nanomedicine can be broadly defined as the use of supramolecular-scale matter under ~100 nanometers to improve therapeutic index. Several existing approved nanotherapies specifically aim at improved delivery of chemo. For example, MM-398, a nanoscale liposomal formulation of the cytotoxic alkaloid irinotecan, was approved in 2015 for metastatic pancreatic cancer [11]. CPX-351 is a liposomal formulation delivering cytarabine and daunorubicin at synergistic molar ratio for acute myeloid leukemia [12].
These examples and others share a common theme: reformulation of non-specific cytotoxic agents, through nanotechnology-enabled delivery to improve ADME (absorption, distribution, metabolism, and excretion) properties. In addition to small size, better biocompatibility, and favorable payload delivery, nanomedicines also take advantage of enhanced permeability and retention (EPR) [13]. While standard chemotherapies enter tissues through passive diffusion, nanocarriers exploit EPR to improve extravasation specifically at tumors, whose vascular beds have increased permeability and dysfunctional lymphatic drainage that facilitate accumulation [13].
These exciting innovations do not yet represent true tumor-targeting precision medicines. Informed by an ever-expanding repertoire of candidate biomarkers, however, addition of targeting moieties to nanotherapies can cross this bridge (Fig. 2) [2, 4]. For example, recent work demonstrated that high expression of the transferrin receptor (TFR1) identifies high-risk DLBCL tumors. This finding was exploited preclinically with a novel nanomedicine created by translational cancer biologists and pharmaco-chemist collaborators, delivering doxorubicin (Dox) to high TFR1-expressing DLBCL tumors (NanoDox) [14]. NanoDox is built on a carbon nitride nanodot (CND) backbone, with co-conjugation of Dox and the TFR1 ligand as a targeting moiety. The molecule showed exponentially increased potency against DLBCL cells in vitro, characterized by homing in on cell-surface tumor TFR1, receptor binding leading to rapid clathrin-mediated internalization, CND-Dox endosomal escape, and delivery of Dox to nuclei, where it chops up cancer genomes by inducing double stranded DNA damage. When switched in for Dox in R-CHOP, creating R-nanoCHOP, it controlled patient-derived xenograft tumors with dramatically reduced toxicity. NanoDox therefore establishes proof-of-concept for targeted delivery to improve chemotherapy efficacy against DLBCL, transforming highly toxic and non-specific Dox (still considered the most active anti-DLBCL agent) into a targeted nanomedicine. The addition of a targeting moiety to the surface of the nanoparticle, in this case a ligand for a biomarker highly-expressed on the surface of high-risk DLBCL tumors but expressed to a lesser extent on non-malignant tissues, is an example of nano-optimized chemotherapy (Fig. 2). Given increased expression on tumor vs. non-tumor cells, CD19, CD20, and CD79b may also prove to be compelling DLBCL targets for nanomedicines. Recent computational approaches driving biomarker discovery can facilitate identification of additional novel targets [2, 4]. We believe this work illustrates an approach with great potential to increase tumor specificity and reduce toxicities to other tissues, providing a two-fold benefit in improving patient outcomes (Fig. 2). Melphanlan flufenamide, FDA approved for multiple myeloma as this article was under preparation, relies on the same principle, exploiting high tumor expression of aminopeptidases. For DLBCL, there is urgent need for more efficacious medicines, specifically, we argue, in the frontline where progress has stagnated [7]. Numerous attempts at improving these treatments have failed and thus the field has shifted focus towards immunotherapies for rel/ref patients (Fig. 1).
While efforts to develop immunotherapies should continue, nano-optimization of chemotherapy offers a different and potentially more easily implemented option. Recent advances in nanotechnologies offer considerable potential to transform drugs like Dox, vincristine, and cyclophosphamide – non-specific chemotherapeutic components of the frontline gold standard R-CHOP – into modern precision medicines. CNDs are highly malleable chemical modalities allowing attachment of multiple different ligands at a variety of molar ratios. Medicinal chemistry optimization could allow, for example, development of tri-chemotherapeutic conjugated precision medicines, designed for maximum on-target efficacy and alleviation of off-target dose-limiting toxicities. Resistance to specific toxic payloads (e.g. monomethyl auristatin E (MMAE)), often used in FDA-approved antibody drug conjugates (ADCs) (brentuximab, polatuzumab), are reported [15]. Nano-chemotherapeutics provide an optimized therapeutic strategy to both overcome such resistance and facilitate rationale clinical implementation by replacing overtly toxic compounds with precision targeting, rather than adding new therapeutic modalities to existing regimens, an approach so far unsuccessful for DLBCL (Fig. 1). The combination of general tumor targeting due to EPR [13] and active tumor targeting via specific biomarkers [14] positions nano-optimized therapeutics to widen therapeutic windows. Despite these potential advantages, however, key questions and challenges remain for successful clinical implementation. Will small molecular size ultimately prove unfavorable for pharmacokinetics? Will this nanotechnology be amenable to efficient manufacturing and formulation? Does increased drug-loading ultimately result in a double-edged sword off-tumor toxicity, as was seen in ADC efforts? These questions must be thoroughly answered in investigational new drug (IND)-enabling nanotherapeutic development campaigns. In sum, nano-optimization is positioned to allow for a transformation of age-old generic medicine into new and improved precision approaches, thus improving outcomes that address unmet needs in DLBCL and potentially many additional deadly cancers.
Acknowledgments
Figures made with BioRender.
References
- 1.DeVita VT Jr. and Chu E, A history of cancer chemotherapy. Cancer Res, 2008. 68(21): p. 8643–53. [DOI] [PubMed] [Google Scholar]
- 2.Chapuy B, et al. , Molecular subtypes of diffuse large B cell lymphoma are associated with distinct pathogenic mechanisms and outcomes. Nat Med, 2018. 24(5): p. 679–690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Waldman AD, Fritz JM, and Lenardo MJ, A guide to cancer immunotherapy: from T cell basic science to clinical practice. Nat Rev Immunol, 2020. 20(11): p. 651–668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Wright GW, et al. , A Probabilistic Classification Tool for Genetic Subtypes of Diffuse Large B Cell Lymphoma with Therapeutic Implications. Cancer Cell, 2020. 37(4): p. 551–568 e14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ennishi D, et al. , Toward a New Molecular Taxonomy of Diffuse Large B-cell Lymphoma. Cancer Discov, 2020. 10(9): p. 1267–1281. [DOI] [PubMed] [Google Scholar]
- 6.Fisher RI, et al. , Comparison of a standard regimen (CHOP) with three intensive chemotherapy regimens for advanced non-Hodgkin’s lymphoma. N Engl J Med, 1993. 328(14): p. 1002–6. [DOI] [PubMed] [Google Scholar]
- 7.Coiffier B, et al. , CHOP chemotherapy plus rituximab compared with CHOP alone in elderly patients with diffuse large-B-cell lymphoma. N Engl J Med, 2002. 346(4): p. 235–42. [DOI] [PubMed] [Google Scholar]
- 8.Elias L, Portlock CS, and Rosenberg SA, Combination chemotherapy of diffuse histiocytic lymphoma with cyclophosphamide, adriamycin, vincristine and prednisone (CHOP). Cancer, 1978. 42(4): p. 1705–10. [DOI] [PubMed] [Google Scholar]
- 9.Philip T, et al. , Autologous bone marrow transplantation as compared with salvage chemotherapy in relapses of chemotherapy-sensitive non-Hodgkin’s lymphoma. N Engl J Med, 1995. 333(23): p. 1540–5. [DOI] [PubMed] [Google Scholar]
- 10.Friedberg JW, Relapsed/refractory diffuse large B-cell lymphoma. Hematology Am Soc Hematol Educ Program, 2011. 2011: p. 498–505. [DOI] [PubMed] [Google Scholar]
- 11.Wang-Gillam A, et al. , NAPOLI-1 phase 3 study of liposomal irinotecan in metastatic pancreatic cancer: Final overall survival analysis and characteristics of long-term survivors. Eur J Cancer, 2019. 108: p. 78–87. [DOI] [PubMed] [Google Scholar]
- 12.Krauss AC, et al. , FDA Approval Summary: (Daunorubicin and Cytarabine) Liposome for Injection for the Treatment of Adults with High-Risk Acute Myeloid Leukemia. Clin Cancer Res, 2019. 25(9): p. 2685–2690. [DOI] [PubMed] [Google Scholar]
- 13.Iyer AK, et al. , Exploiting the enhanced permeability and retention effect for tumor targeting. Drug Discovery Today, 2006. 11(17): p. 812–818. [DOI] [PubMed] [Google Scholar]
- 14.Arumov A, et al. , Optimized doxorubicin chemotherapy for diffuse Large B-Cell lymphoma exploits nanocarrier delivery to transferrin receptors. Cancer Res, 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Chen R, et al. , CD30 Downregulation, MMAE Resistance, and MDR1 Upregulation Are All Associated with Resistance to Brentuximab Vedotin. Mol Cancer Ther, 2015. 14(6): p. 1376–84. [DOI] [PMC free article] [PubMed] [Google Scholar]


