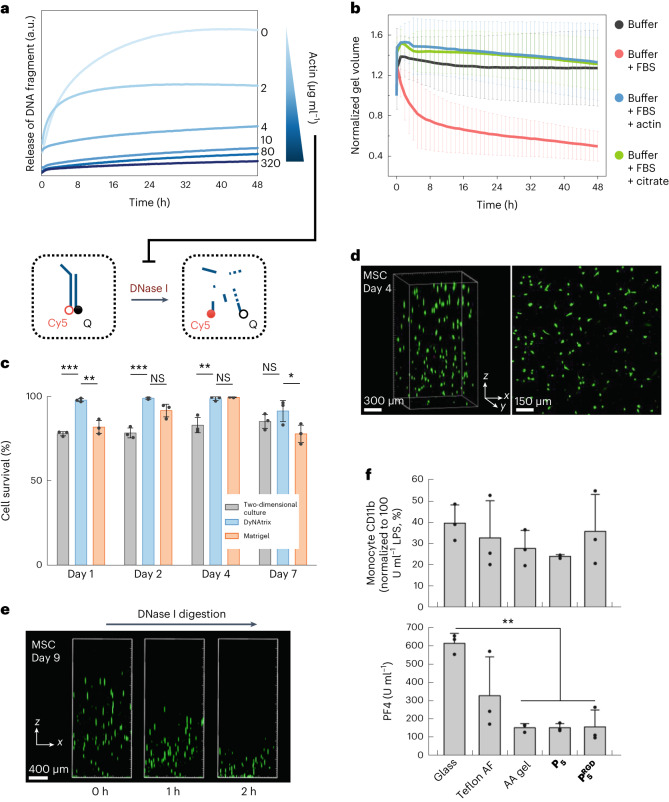
Fig. 5. DyNAtrix exhibits tunable stability against degradation in cell culture, high biocompatibility and DNase I-mediated release of live cells.
a, Digestion experiment. A digestion probe containing a fluorophore (Cy5) and a fluorescence quencher (Q) was incubated in medium containing DNase I and different concentrations of actin. Duplex digestion leads to dissociation of the strands and increased Cy5 emission. b, Normalized volume of DyNAtrix in FBS-supplemented medium over time. Samples were incubated in buffer (negative control; black) or serum-containing medium without protection (red). The addition of actin (50 µg ml−1; blue) or citrate (10 mM; green) effectively protected the gels from digestion. Data are shown as mean ± s.d. (n = 4). c, Viability of MSCs cultured in actin-protected DyNAtrix compared with Matrigel and a two-dimensional culture plate. Data are shown as mean ± s.d. (n = 3). Statistical analysis was performed using one-way ANOVA followed by a Tukey post hoc test. NS, not significant; *P < 0.05; **P < 0.01; ***P < 0.001. d, Live/dead staining of MSCs in DyNAtrix (1% (w/v) + CCL-64) after 7 days in culture. Live cells were stained with calcein AM (green) and dead cells were stained with propidium iodide (pink). e, Release of live MSCs from DyNAtrix under mild conditions via the addition of DNase I on day 9. f, Quantification of DyNAtrix-triggered immune response and haemocompatibility. Top: activation of monocytes in human whole blood was measured by flow cytometric quantification of CD11b expression. Bottom: platelet activation was assessed by quantification of the haemostasis marker platelet factor 4 (PF4). CCL-64-crosslinked 1% (w/v) P5 and were compared with 3 reference substrates: reactively cleaned glass, polytetrafluoroethylene (Teflon AF) and covalently crosslinked polyacrylamide (AA) gel. Data are shown as mean ± s.d. (n = 3). Statistical analysis was performed using one-way ANOVA followed by a Holm–Sidak post hoc test (**P < 0.01).