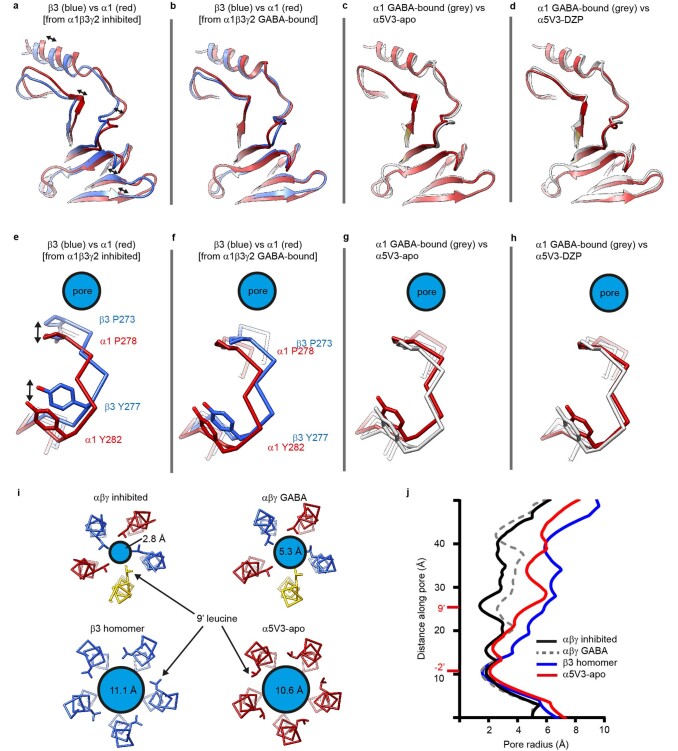
Extended Data Fig. 7. α5V3 conformation.
a-d, Structural models represented as ribbon diagrams looking down on the upper portions of subunit extracellular domains. Overlay comparing the superposition of the β3 and α1 subunits in α1β3γ2 inhibited by bicuculline, a, show that the α1 subunit is twisted into the activated conformation relative to the β3 subunit, whereas in the presence of GABA, b, the β3-subunit becomes activated and rotates to assume the same twisted arrangement as the α1 subunit. c-d, Overlays of the α1 subunit versus α5V3, show that the α5V3-apo, c, and α5V3-DZP, d, subunits match the activated twisted α1 subunit arrangement observed in inhibited and GABA-bound α1β3γ2 receptors. e-h, Comparisons of the same subunit pairings, but this time from the perspective of the position of the M2-M3 loop in Cα stick representation relative to the pore. Overlay comparing the superposition of the β3 and α1 subunit M2-M3 loops in α1β3γ2 inhibited by bicuculline, e, show that the α1 subunit is retracted from the pore relative to the β3 subunit, whereas in the presence of GABA, f, the β3-subunit also retracts. g-h, Overlays of the α1 subunit versus α5V3, show that the α5V3-apo, g, and α5V3-DZP, h, are also retracted. i, Cross sections of the pore showing positions of hydrophobic activation gate 9’ leucines and pore diameters showing that inhibited α1β3γ2 is closed, GABA-bound α1β3γ2 is open, and the β3 homomer and α5V3 are even more open. j, Pore radius plots. Inhibited α1β3γ2 receptor (bound by antagonist bicuculline) is PDB 6HUK; GABA-bound α1β3γ2 receptor (also bound by diazepam) is PDB 6HUP; β3-homomer is PDB 4COF.