Figure 1. US academic centers involved in AD subject recruitment and Study Schematic for the Atopic Dermatitis Research Network (ADRN) 09 study (NCT03389893).
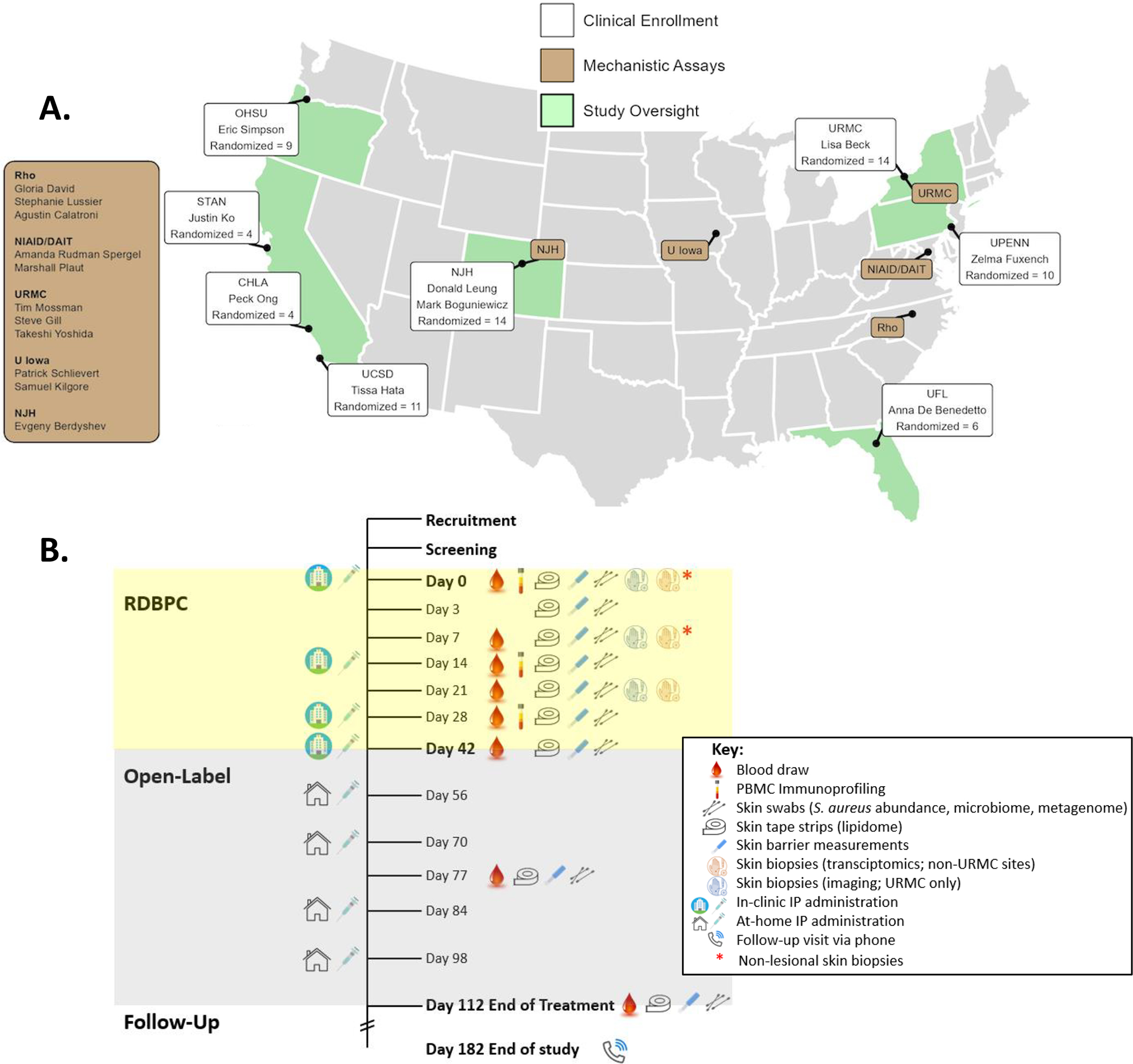
(A) A map of the US academic centers involved in clinical enrollment (white boxes) or mechanistic assays or study oversight (brown boxes). Each white box represents a single center with investigator(s) and enrollment totals. OHSU=Oregon Health & Science University, STAN=Stanford Medical Center, CHLA=Children’s Hospital of Los Angeles, UCSD=University of California San Diego, NJH=National Jewish Health, URMC=University of Rochester Medical Center, UPENN=University of Pennsylvania, UF=University of Florida. (B) High density sampling of the microbial, epithelial, and immune compartments was performed during the 6-week RDBPC phase (yellow) of the trial to characterize and quantify changes and their relationship to disease improvements, followed by a 10-week open-label extension (OLE; grey) phase with a safety assessment (by phone) 10 weeks after the OLE phase ended. ∗Timepoints when non-lesional skin biopsies were collected in addition to lesional biopsies for transcriptomics analysis.
