Figure 2. Dupilumab treatment improves all measures of AD severity.
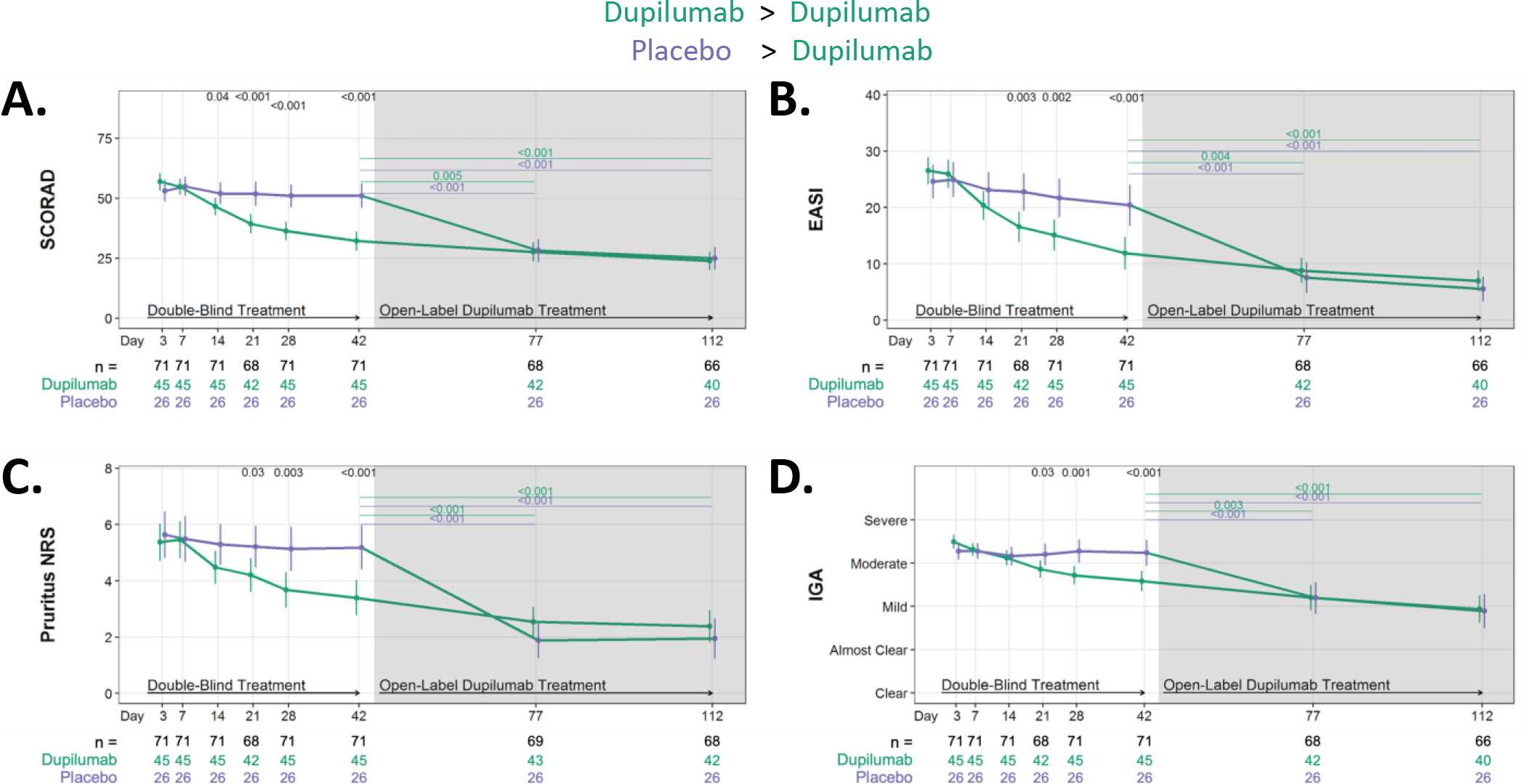
Absolute changes in AD severity measures during RDBPC phase (up to day 42 [6 weeks]) and during the OLE phase (day 42 – day 112 or [6 – 16 weeks]). (A) Absolute reductions in SCORing AD (SCORAD), (B) Eczema Area and Severity Index (EASI), (C) Pruritus Numerical Rating Scale (NRS) and (D) Investigator Global Assessment (IGA). Data for A, C, and D are shown as the means and 95% CIs adjusted for clinical site and EASI (≥ 21.1 or < 21.1), and the severity measure at day 0. Data for B is shown as the means and 95% CIs adjusted for clinical site and EASI at day 0. The number of participants with evaluable data at each timepoint are noted below the X axis (with the total population denoted in black, dupilumab-randomized participants in green and placebo-randomized in purple).
