Figure 3. Dupilumab treatment rapidly reduces S. aureus abundance and cytotoxin production on the skin surface of AD participants.
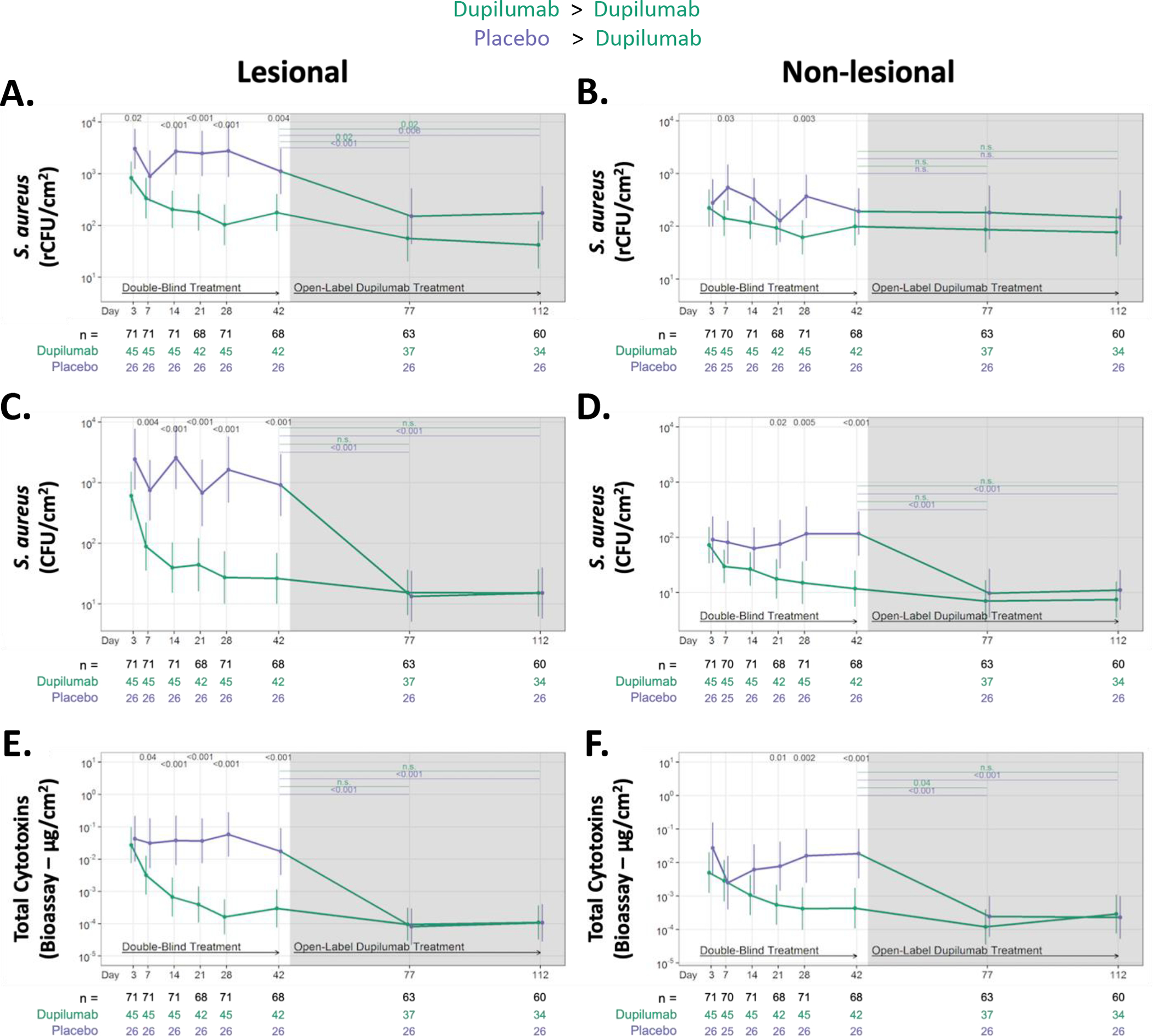
Absolute changes in S. aureus abundance and cytotoxin production during RDBPC (up to day 42 [6 weeks]) and OLE phases (6 – 16 weeks) on lesional (left) and non-lesional (right) skin. S. aureus abundance was measured using qPCR for the femA gene (rCFU/cm2) in both lesional skin (A) and non-lesional skin (B) and by quantitative culture techniques (CFU/cm2) in both lesional skin (C) and non-lesional skin (D). Total S. aureus cytotoxin levels (μg/cm2), measured by bioassay from skin swabs of both lesional (E) and non-lesional skin (F), are shown. Data are shown as geometric means and 95% CIs and are adjusted for clinical site, disease severity at day 0, as measured by EASI ≥ 21.1 or < 21.1, and S. aureus abundance on lesional skin at day 0. The number of participants with evaluable data at each timepoint are noted below the X axis (with the total population denoted in black, dupilumab-randomized participants in green and placebo-randomized in purple). rCFU, relative colony-forming units
