Abstract
When S-layered strains of Bacillus stearothermophilus and Aneurinibacillus thermoaerophilus, possessing S-layers of different lattice type and lattice constant as well as S-(glyco)protein chemistry, and isogenic S-layerless variants were subjected to membrane vesicles (MVs) from P. aeruginosa during plaque assays on plates or CFU measurements on cell suspensions, all bacterial types lysed. Electron microscopy of negative stains, thin sections, and immunogold-labelled MV preparations revealed that the vesicles adhered to all bacterial surfaces, broke open, and digested the underlying peptidoglycan-containing cell wall of all cell types. Reassembled S-layer did not appear to be affected by MVs, and sodium dodecyl sulfate-polyacrylamide gel electrophoresis analysis showed that the S-(glyco)proteins remained intact. meso-Diaminopimelic acid, as a peptidoglycan breakdown product, was found in all culture supernatants after MV attack. These results suggest that even though MVs are much larger than the channels which penetrate these proteinaceous arrays, S-layers on gram-positive bacteria do not form a defensive barrier against the lytic action of MVs. The primary mode of attack is by the liberation from the MVs of a peptidoglycan hydrolase, which penetrates through the S-layer to digest the underlying peptidoglycan-containing cell wall. The S-layer is not affected by MV protease.
S-layers are planar paracrystalline assemblies which coat the surfaces of many gram-negative and gram-positive bacteria (eubacteria) and archaea (archaeobacteria) (4, 5, 23, 28–30). The subunits of these layers are thermodynamically driven to self-assemble, and hexagonal (p6), square (p4), trimeric (p3), and oblique (p1, p2) crystal lattices are possible (4, 5, 23, 28–30). Although most S-layers are made of protein, some which possess glyco-substituents have been found (20, 22). As self-assembly occurs at the surface of the bacterium, S-(glyco)proteins reconfigure themselves, as they interact with one another and the underlying cell wall, to form a minimum energy structure (the S-layer) held together by noncovalent bonds, such as salt-bridging (often involving Ca2+), ionic-bonding, hydrogen-bonding, and hydrophobic interaction. Once the S-layer has formed, its outer face is typically more hydrophobic than the uncoated cell wall (4, 30). Yet, because this structure consists of regularly arranged subunits, there exists a network of identically sized pores permeating the S-layer capable of selectively filtering macromolecules according to size, shape, and charge (27, 30). When present as structural components of pathogenic bacteria, S-layers contribute to virulence (8, 9, 13, 19, 25).
Recently we have reported that during normal growth, Pseudomonas aeruginosa produces membrane vesicles (MVs) filled with periplasmic components, including hydrolytic enzymes such as protease, phospholipase C, and peptidoglycan hydrolase (14). Accordingly, MVs are small (diameter, ∼30 to 50 nm) bilayered particles into which degradative enzymes are concentrated. It is possible that MVs have a predatory role in natural ecosystems, in which they are released by a parent bacterium so as to lyse surrounding cells, increasing available nutrients to the parent strain. Certainly, MVs are capable of lysing a variety of gram-positive and gram-negative bacteria (15). For gram-positive cells, MVs adhere to the cell wall, where they break open and digest the immediate underlying peptidoglycan. For gram-negative bacteria, MVs fuse into the outer membrane, releasing their contents into the periplasmic space for dispersal around the cell so that the peptidoglycan sacculus can be hydrolyzed at several points.
Taken in the context of predation, it is possible that one strategy for gram-positive bacteria to avoid lysis by MVs would be to construct an additional protective layer above their cell walls. Because these same bacteria would still have to gain nutrients and excrete wastes by diffusion, the protective layer would have to be porous. Also, because MVs are deformable and (presumably) capable of threading their way through thixotropic structures such as capsules, the layer would have to be a rigid matrix in which the pore size is accurately controlled. Macromolecules on the order of 40 to 60 kDa could penetrate, but larger particulate debris could not (27). All of these features conform to the attributes of S-layers (1, 4, 6, 29). It was therefore necessary to test MVs against well-characterized gram-positive bacteria possessing S-layers in p2, p4, and p6 arrays which consisted of either protein or glycoprotein. For this reason, in our study we have used different Bacillus stearothermophilus and Aneurinbacillus thermoaerophilus strains possessing well-defined lattices composed of well-characterized S-(glyco)proteins as well as their S-layerless variants.
(Part of this work was previously reported in a poster session at the 1997 General Meeting of the American Society for Microbiology in Miami Beach, Fla.)
MATERIALS AND METHODS
Description and growth of bacterial strains.
B. stearothermophilus PV72/p6 was kindly provided by F. Hollaus (Zuckerforschung, Tulln, Austria) and possesses an S-protein (Mr = 130 kDa) in a hexagonal lattice with a center-to-center spacing of 22.5 nm. Strain PV72/p2 is an oxygen-induced variant strain derived from PV72/p6 (26) and possesses an S-protein (Mr = 97 kDa) in an oblique lattice with a = 9.7 nm, b = 7.6 nm, and γ = 81°. Both strains were grown in 50 to 100 ml of either Nutrient Broth (Difco) or S-VIII medium (1) containing 5 g of yeast extract, 10 g of peptone, and 5 g of Lab Lemco per liter in 300- to 500-ml flasks at 57°C to midexponential growth phase. Strain S65-67 (now abbreviated S65) has an S-protein (Mr = 84 kDa) in a square lattice with center-to-center spacing of 10.0 nm and was grown as described above. Strain PV72/T5 is a natural isogenic S-layerless mutant of PV72, whereas E23-67 (now abbreviated E23) is an unrelated S-layerless strain; both were grown under conditions similar to those for the S-layered strains and are used as controls. A. thermoaerophilus is a new species which produces an S-glycoprotein and has recently been described by Meier-Stauffer et al. (18). A. thermoaerophilus DSM 10155 (abbreviated 10155/C+, where C stands for the glycosylated S-layer protein), which has an S-glycoprotein [Mr = 153,000] in a square lattice with center-to-center spacing of 10.0 nm, and A. thermoaerophilus 10155/C− (a spontaneous variant of DSM 10155 which does not possess the glyco-substituent on its S-protein but which has the same lattice constant) were also used in the study.
Preparation of P. aeruginosa MVs.
These were produced, isolated, and purified exactly according to the method of Kadurugamuwa and Beveridge (14). Protease, phospholipase C, and peptidoglycan hydrolase activities of the MVs used in this study were also monitored according to their techniques (14).
Lytic activity of MVs on B. stearothermophilus and A. thermoaerophilus strains. (i) Plaque assay on plates.
The bacterial strains were grown in S-VIII medium overnight and adjusted to an optical density at 470 nm of 0.25 in fresh medium. Agar plates (5% agar) prepared with S-VIII medium were overlaid with 1.0 ml of bacterial suspension which was spread uniformly on agar surface and left at room temperature until the surface became dry. A 20-μl aliquot of an MV preparation (a total of 20 μg of MV protein) was dropped onto the agar surface and incubated for 16 h at 60°C. Spots showing zones of clearing were considered to have lytic activity for the strain under investigation.
(ii) Bacteriolytic activity determined by CFU.
The lytic activity of MVs was determined by using PV72/p2, S65, PV72/p6, 10155/C+, 10155/C−, PV72/T5, and E23 strains as test organisms. Exponential-growth-phase cultures were diluted in fresh S-VIII broth to produce a bacterial suspension of 108 CFU/ml. At zero time, MVs (50 μg of protein per ml) were added to the cultures and incubated at 37°C. We estimated that at this MV concentration there would be approximately one MV per 10 bacteria, and this estimation was confirmed by electron microscopy. The bactericidal activity was monitored by viable counting at various times (0 to 2 h) on S-VIII agar medium after incubating the plates for 16 h at 37°C.
(iii) Detection of soluble DPM from peptidoglycan in supernatants after treatment of cells with MVs.
PV72/p2, S65, PV72/p6, 10155/C+, 10155/C−, PV72/T5, and E23 cell pellets (1.0 mg [wet weight]) were incubated for 2 h at 37°C with 50-μg protein samples of MVs in a total volume of 100 μl of 0.02 M Tris-HCl (pH 8.0). The suspensions were centrifuged at 6,000 × g for 15 min. All culture supernatants were hydrolyzed with 200 μl of 2 M hydrochloric acid at 100°C for 2 h. Controls consisted of cell pellets that had not been exposed to MVs but which were incubated with or without 2 M HCl at 100°C for 2 h. After incubation, HCl was removed from the reaction mixtures by leaving the samples in a desiccator under diminished pressure and at an elevated temperature for 2 to 3 h. The samples were resuspended in 50 μl of distilled water, and each sample was spotted onto a thin-layer chromatography (TLC) plate (Silica Gel 60 particle size, 5 to 17 μm; Sigma). Authentic αɛ-meso-diaminopimelic acid (DPM) (Sigma) was used as a standard for the quantification of the amount of DPM released from hydrolyzed peptidoglycan. The hydrolysates were separated on TLC plates with a mixture of butanol-acetic acid-water (4:1:1) as described previously (15). After chromatography, the plates were sprayed with 0.02% ninhydrin (33) and the DPM content was estimated densitometrically by scanning the plates in the Bio-Rad Gel Doc 1000 system (Bio-Rad, Richmond, Calif.). Peak assessment was performed manually after background subtraction, and the amount of DPM in each sample was calculated.
Effect of MVs on S-layers.
All S-(glyco)proteins have been well characterized from all the S-layered bacilli used in this study (24, 26) so that sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) of boiled 2% (wt/vol) SDS extracts of the various fractions after experimentation could be used to detect alterations of Mr in S-(glyco)proteins due to proteolysis by the MVs. For this approach, the SDS-PAGE banding patterns of supernatants from the CFU experiments were compared to the banding patterns derived from the cells used in each experiment as well as from untreated control cells. In addition, S-layers extracted from each strain by 5 M guanidine hydrochloride (in Tris-HCl buffer, pH 7.2) treatment (16, 21) were reassembled on S-layer-deficient cell walls from each parent strain by adding 50 μg of S-(glyco)protein to 50 μg (dry weight) of cell walls and dialyzing in distilled water overnight at 4°C. In some experiments, peptidoglycan-containing sacculi were used as reassembly templates; these sacculi were derived from isolated cell walls that were boiled in 2% (wt/vol) SDS for 30 min and washed free of SDS (32). These preparations were also subjected to MVs (10 μg of protein/ml of wall suspension [optical density at 600 nm, 0.2]) for 0.25 to 6.00 h at room temperature before SDS-PAGE analysis. Both PV2/p6 and PV2/p2 are capable of having their S-layers reassemble on their own without a cell wall interface. For this experiment, the S-protein was extracted with 5 M guanidine hydrochloride (as described before) to solubilize it. This S-protein-containing supernatant was centrifuged at 100,000 × g for 2 h to pellet particulate matter, and the supernatant was dialyzed at 4°C overnight in distilled water. The S-protein in the dialysate reassembled into opalescent flocs, which were separated from the fluid by centrifugation at 40,000 × g and resuspended into small fragments by vortexing as new fluid was added. The integrity of the reassembled S-layer was monitored by electron microscopy, and the preparation was subjected to MVs (10 μg of protein/1 mg of S-protein) for 0.25 to 6.00 h at room temperature before SDS-PAGE analysis. For all experiments, the S-layerless variants (PV72/T5 and E23) served as negative controls.
Transmission electron microscopy (TEM).
A combination of negative stains, freeze-etching, conventional thin sections, freeze-substitution thin sections, and immunogold labelling of thin sections and whole mounts was used to monitor all aspects of the MV experiments. For detailed procedures of these techniques, see the methods of Beveridge et al. (7). For negative strains, 2% (wt/vol) uranyl acetate in water was used. For freeze-etching, platinum was vaporized as the shadowing agent at an angle of 45°. For conventional thin sections, the cells were chemically fixed in 2% (vol/vol) glutaraldehyde followed by 2% (wt/vol) osmium tetroxide by using 50 mM HEPES, pH 6.8, as a buffer. Before the OsO4 fixation, the cells were enrobed in 2% (wt/vol) Noble agar. En bloc staining was performed in 2% (wt/vol) uranyl acetate. The preparations were dehydrated through an ethanol series and infiltrated with LR white resin, which was cured at 60°C for 1 h. Freeze-substitutions were performed after freeze-plunging the cells according to the method of Graham and Beveridge (10). The freeze-substitution mixture consisted of 2% (wt/vol) uranyl acetate and 2% (wt/vol) OsO4 in acetone containing a molecular sieve to ensure dryness (11) and was held at −80°C until substitution was complete. After this, cells were infiltrated with LR white or Epon 812 and cured. All sections were cut using a Reichardt Ultracut E microtome using a diamond knife.
MVs and their attachment to bacteria were monitored by the indirect immunogold labelling procedure (7). Whole mounts or thin sections of cells treated with MVs were first incubated with monoclonal antibodies specific for the B-band lipopolysaccharide (LPS) of P. aeruginosa followed by protein A-gold (14). Skim milk was used as an unspecific blocking agent, and protein A-gold (alone) was used as a negative control.
For all preparations, TEM was done with either a Philips EM300 or EM400T under standard conditions at 60 kV with the anticontaminators in place.
RESULTS
Ultrastructure of the cell envelopes of the bacilli used in this study.
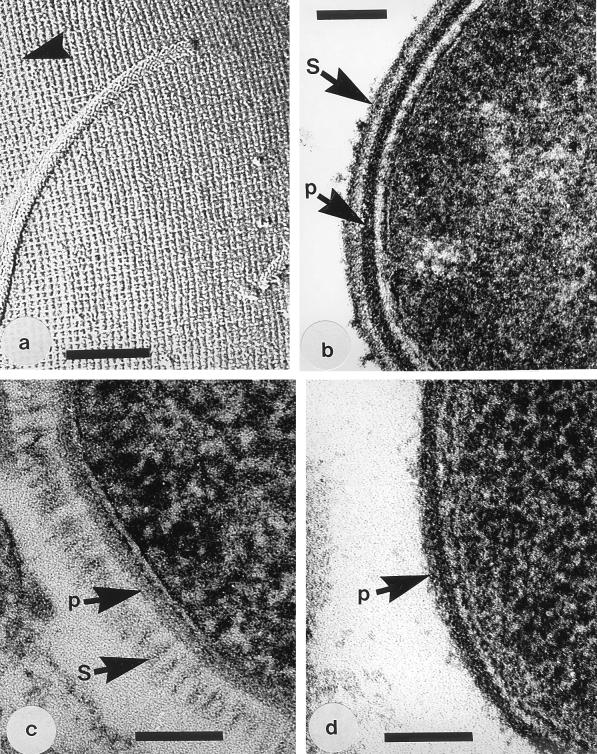
All but the S-layer of B. stearothermophilus PV72/p6 could be readily visualized in negative stains or freeze-etchings (Fig. 1a) and conventional thin sections (Fig. 1b), and these preparations verified the spacing and symmetry aspects of the S-layers. When freeze-substituted, the S-layer of strain PV72/p6 was well preserved and showed the 22.5-nm spacing of hexagonally arranged subunits (Fig. 1c). Thin sections of the S-layer-deficient variants verified that no S-layer was present (Fig. 1d).
FIG. 1.
(a) Image of the freeze-etched surface of A. thermoaerophilus 10155/C+ showing the p4 lattice (a = b = 10 nm) of its S-glycoprotein. The arrowhead shows the direction of the platinum shadow. The magnification bar in this and the following panels represents 100 nm. (b) Thin section of a conventionally fixed B. stearothermophilus PV72/p2 showing the peptidoglycan layer (p) and S-layer (S). (c) Thin section of a freeze-substituted B. stearothermophilus PV72/p6 which shows the S-layer (S) as a regularly arranged system of fibers emanating from the peptidoglycan layer (p). This S-layer was seen neither in negative stains nor conventional embeddings of whole cells. (d) Thin section of a conventionally fixed B. stearothermophilus PV72/T5, which is an S-layerless variant (26), showing only the peptidoglycan-containing layer (p). Similar images of this S-layerless variant were produced by the freeze-substitution method.
TEM of bacilli after treatment with P. aeruginosa MVs.
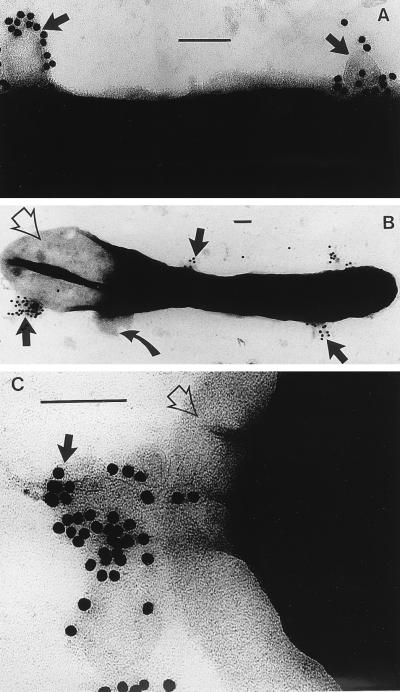
Because MVs were capable of lysing all bacilli used in this study (see below), it was apparent that it would be difficult to distinguish P. aeruginosa MVs from membranous debris derived from the plasma membrane of lysed cells. For this reason, we relied on detecting the MVs by immunogold labelling of the serotype (B-band) LPS which they expressed on their bilayer surface (Fig. 2A). In fact, the two types of membranes could also be distinguished from one another by size; MVs have a consistent small diameter of 30 to 50 nm (14), whereas the plasma membrane vesicles extruding from lysed cells were twofold (or more) larger (Fig. 2B). In these whole mounts of MV-treated cells, it was apparent that the cell wall was collapsing close to extrusion regions, which was indicative of cell lysis as the cytoplasm departed from the protoplast (Fig. 2C).
FIG. 2.
(A) Negative stain of the surface of B. stearothermophilus PV72/p6 immediately after MVs have been added to the cells. The MVs have been labelled with an anti-B-band LPS immunogold which is specific for the MVs (14); the MVs have attached to the surface of the bacterium (arrows). The magnification bar in this and the following panels represents 100 nm. (B) An entire B. stearothermophilus cell after 10 min of MV treatment is shown. Large membranous vesicles (those not labelled with colloidal gold), derived from the bacterium’s plasma membrane, are seen extruding from the cell where the peptidoglycan has been hydrolyzed (curved arrow). One pole of the bacterium has been emptied of cytoplasm (empty arrow). MVs (labelled with gold) are still attached to the bacterium (solid arrows). (C) High magnification of the pole of a cell which is extruding cytoplasm since the peptidoglycan layer has been hydrolyzed (empty arrow). MVs and the gold label still remain attached (black arrow).
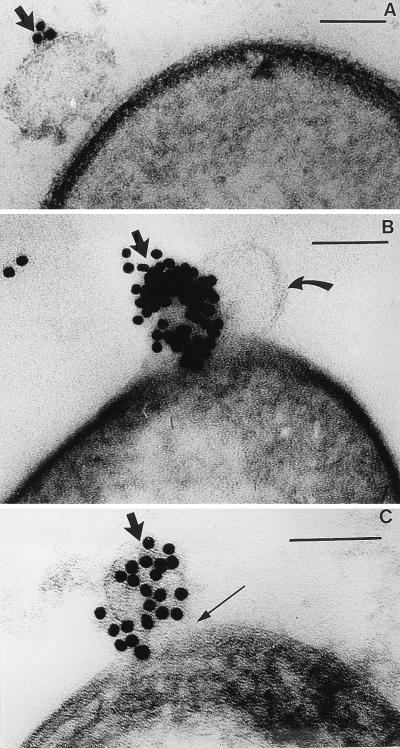
Thin sections were even more informative, since they clearly demonstrated that the MVs adhered to the cell surface (Fig. 3A), broke open (thereby liberating their luminal contents) (Fig. 3B), and digested the underlying peptidoglycan-containing cell wall (Fig. 3C). From these preparations, it was apparent that the S-layer remained intact while the peptidoglycan was hydrolyzed immediately below the zone of MV attachment.
FIG. 3.
(A) Thin section of B. stearothermophilus PV76/p2 showing the initial stage of MV attachment to the bacterial surface. The arrow points to a gold-labelled MV. The magnification bar in this and the following panels represents 100 nm. (B) The MV in this image has lysed the cell after 20 min, and a balloon of cytoplasm, bounded by the plasma membrane (curved arrow), is extruding from the cell. The plasma membrane is not labelled, but an adjacent MV (solid arrow) is labelled by its immunogold probe. (C) In this image, the gold-labelled MV (thick arrow) has completely hydrolyzed the peptidoglycan layer (thin arrow) after 30 min.
Plaque assays on plates and CFU.
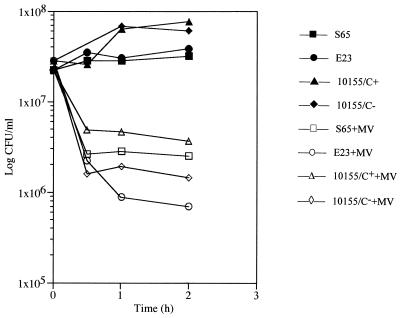
When suspensions of MVs were spotted on plates of all S-layered bacilli and the deficient variants, lysis occurred wherever the MVs were dropped. This was confirmed when cell suspensions were treated with MVs and the suspensions were plated out to determine CFU. It was apparent that MVs were killing all of the S-layered and the S-layerless strains (Fig. 4). Those strains possessing S-layers seemed to be slightly less affected than the S-layer-deficient variants.
FIG. 4.
The killing effect of MVs on B. stearothermophilus and A. thermoaerophilus strains is shown by comparing the number of CFU per ml over a 2-h treatment (open symbols). When compared with the same strains without MV treatment (solid symbols), it is apparent that MVs lyse and kill the bacteria. Strains E23 and 10155/C− do not have S-layers and were slightly more sensitive to the MVs than strains S65 and 10155/C+, which possess p4 S-layers.
Detection of DPM.
From the foregoing experiments, it seemed apparent that the major cause of death was cell lysis due to peptidoglycan digestion by the peptidoglycan hydrolase contained in the MVs. For this reason, cell cultures were examined by the method of Work (33) for soluble DPM in the supernatant after they were treated by MVs. Since this colorimetric assay cannot distinguish between diamino acids, the supernatants were concentrated and run on TLC plates to identify DPM. For this experiment, S65 and E23 were focused on (as the S-layered strains) and the assays were done in triplicate during three separate experiments. Cells which were not treated with MVs served as controls. For S65, 18.2 ± 1.4 μg of DPM per mg of cells (wet weight) was liberated by the MVs, whereas only 2.8 ± 0.8 μg of DPM per mg was liberated from untreated cells (results are means ± standard errors of the means). For E23, 26.4 ± 1.8 μg of DPM per mg was liberated versus 2.9 ± 0.4 μg of DPM per mg for untreated cells. We attribute the low background levels of DPM from the control cells to normal cell wall turnover. A similar trend was detected in the amount of DPM released after treatment of the other strains with MVs, but in these cases, the background DPM in the supernatants from cells which were not treated with MVs was below detectable levels by our procedures. In all cases, MVs produced soluble DPM in the supernatants which confirmed the peptidoglycan hydrolase activity of the MVs on the cells. Presumably, this activity lysed and killed the cells.
Effect of MVs on S-layers.
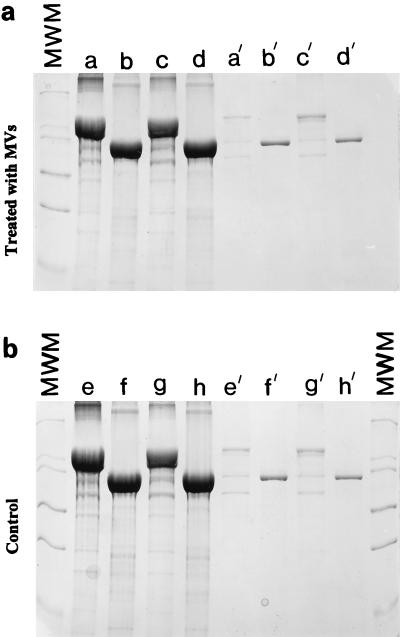
Figure 5a shows the S-proteins derived from S-layered cell wall fragments of PV72/p6 and PV72/p2 by guanidine hydrochloride extraction and their reassembly products either on hot SDS-isolated peptidoglycan-containing sacculi or alone (26). They form the major band in the gel, and their Mrs compare favorably with those that have been previously published. Figure 5b shows the same products after MV treatment. No S-layer degradation products were seen. It was apparent that the protease of the MVs did not break up the S-layer or degrade the S-protein of each PV2/p6 or PV2/p2.
FIG. 5.
SDS-PAGE of S-proteins derived from S-layered cell wall fragments of PV72/p6 (lanes a, c, e, and g) and PV72/p2 (lanes b, d, f, and h) by guanidine hydrochloride extraction and their reassembly products either on hot SDS-treated peptidoglycan-containing sacculi (lanes a, b, e, and f) or alone (lanes c, d, g, and h). (a) Pellets after incubation with MVs; (b) controls (pellets incubated in buffer). The darkest-staining bands in all lanes are the S-proteins. About 10% of the S-layer protein was detected in the clear supernatants after centrifugation of the suspension (lanes with prime symbols). The amount of S-layer protein detected in the supernatant was independent of the incubation in buffer or the use of MVs (i.e., 10% is the usual amount which is solubilized if assembled S-layer material is incubated in buffer under neutral pH conditions). It is clear that the MVs are not digesting any of the S-protein reassemblies. MWM, molecular weight markers.
DISCUSSION
The blebbing of MVs from P. aeruginosa is a natural phenomenon which occurs on free-living cells growing in a variety of growth media, whether it be solid or broth (15a). This phenomenon is also found in biofilms (6, 31). Remarkably, MVs contain high concentrations of potent virulence factors, such as serotype LPS, protease, phospholipase C, and proelastase (14). It is possible that P. aeruginosa, as an opportunistic pathogen, uses MVs as an additional secretion pathway whereby these factors can be concentrated and packaged from the periplasm so that they can be targeted to the tissue before infection. MVs that contain serotype LPS on the outer face of their bilayers are readily engulfed by tissue cell lines, thereby allowing the virulence factors easy entry and activity in tissue while the pathogen remains outside of the cells awaiting its attack (15a). Presumably, this helps facilitate infection and mediate complete infection.
Yet, because P. aeruginosa is a ubiquitous bacterium found in many different environments outside human or animal tissue, MV production as a general trait may have additional purposes. Because MVs also contain the major 26-kDa autolysin (peptidoglycan hydrolase) of the bacterium (17), they also have the ability to lyse a number of gram-positive and gram-negative eubacteria; for this reason we have coined the term “predatory MVs” for them (15). It is our belief that when P. aeruginosa is growing under nutrient-limiting conditions in natural ecosystems, MVs can attack neighboring bacteria, lyse them, and provide the parent strain with complex nutrients on which to feed (6). For gram-positive cells the lytic effect on MVs is, so far, ill defined other than that MVs attach to the cell wall and break open the wall material immediately below them (15). MVs lyse gram-negative bacteria by fusing into their outer membranes and liberating the peptidoglycan hydrolase into the periplasmic space where it can attack the peptidoglycan sacculus at a number of different sites (15). Based on our unpublished observations of samples from a number of natural settings and laboratory simulations using gram-negative bacteria as planktonic and biofilm populations, P. aeruginosa intermingles with unrelated bacteria as well as with closely related daughter cells and liberates MVs against them all. Daughter cells would be unable to stop MVs from fusing into their outer membranes or stop the autolysin from entering their periplasms. But, because the autolysin is “self,” it would enter the existing autolysin pool and would be regulated by the daughter cell. It would not cause indiscriminate lysis. Yet, unrelated bacteria that could not easily regulate this potent enzyme would readily lyse.
In another publication, we have looked at the lytic potency of P. aeruginosa MVs on rather simple gram-negative and gram-positive bacteria; i.e., an Escherichia coli strain, an aminoglycoside-impermeable P. aeruginosa strain (not closely related to our PAO1 MV-producing strain), and a Staphylococcus aureus strain (15). At that time, we saw lesions in the gram-positive cell wall and gram-negative murein sacculus after MVs had attacked cell cultures but the bona fide identification of solubilized peptidoglycan constituents was not done (as it has been in the present publication). We also recognize that many bacteria, when looked at in their natural settings, possess additional surface layers (such as capsules, sheaths, and slimes) above their walls (2). These additional layers may be impediments to the lytic action of MVs since the vesicles may not be able to gain access to the cell wall. Since all three external structures consist of a variety of homo- and heteropolymers which are highly hydrated, they are considered to be thixotropic, thereby undergoing easy gel-to-liquid phase transition depending on the external chemical environment and energy load (2, 3). For this reason, these three external structures may not be good physical barriers to MVs. Instead, S-layers would be a more obvious barrier because they are much more rigid structures with a definite porosity (27). These layers separate the cell wall from the external milieu while maintaining open conduits between their constituent subunits for the passage of diffusive substances of intermediate molecular weight (27). Indeed, natural environments nurture the occurrence of S-layered bacteria, and it is common for S-layers to be readily lost once isolates undergo serial culturing in the laboratory (2, 16, 26). It is possible that one function for the existence of S-layers is to protect the bacterium from the predatory attack by MVs. This was the rationale used to determine the importance of the effect of MVs on S-layered bacilli.
Our experimentation in this article demonstrates that, although S-layers do not allow MVs to pass through their interstices, they cannot protect the cell from MV predation. MVs adhere to the S-layers, break open, and release their peptidoglycan hydrolase, which digests the underlying peptidoglycan-containing cell wall. In fact, the CFU experiments suggest that there is little difference between their killing action on S-layered cells and that of the isogenic S-layerless variants. Interestingly, the S-layer itself does not appear to be sensitive to the action of the MV protease. Although we cannot preclude the possibility of localized proteolytic attack by MVs at S-layer discontinuities (e.g., growth sites) (12), this was not extensive enough to be easily detected in gels and we could not detect such activity by electron microscopy. Resistance to proteolytic attack is not unusual, since many S-(glyco)proteins resist a number of commercial proteases once they have assembled into an S-layer (16, 22).
As our research on MVs from other cells progresses, we find that the production of MVs is a general trait of most gram-negative eubacteria. We are currently characterizing these other MVs and may find that a proportion also contains peptidoglycan hydrolases which can lyse other bacteria. Therefore, so-called predatory MVs could be a more universal phenomenon in the microbial world than was previously thought.
ACKNOWLEDGMENTS
A.M. visited T.J.B.’s laboratory from the Universität für Bodenkultur, Vienna, Austria, under the bilateral exchange agreement between T.J.B.’s and U.B.S.’s universities; she was funded by a stipend from the Österreichische Akademie der Wissenschaften. This research was funded by grants to T.J.B. from the Canadian Bacterial Disease Network National Centres of Excellence Program and the Natural Sciences and Engineering Research Council of Canada (NSERC) and grants to P.M., M.S., and U.B.S. from the Austrian Science Foundation (S7201-MOB, S7202-MOB, and S7205-MOB, respectively). The electron microscopes in the NSERC Guelph Regional STEM Facility are partially maintained by an NSERC Major Facilities Access Grant to T.J.B.
REFERENCES
- 1.Bartelmus W, Perschak F. Schnellmethode zur Keimzahlbestimmung in der Zuckerindustrie. Z Zuckerind. 1957;7:276–281. [Google Scholar]
- 2.Beveridge T J. Ultrastructure, chemistry, and function of the bacterial wall. Int Rev Cytol. 1981;72:229–317. doi: 10.1016/s0074-7696(08)61198-5. [DOI] [PubMed] [Google Scholar]
- 3.Beveridge T J. The bacterial surface: general considerations towards design and function. Can J Microbiol. 1988;34:363–372. doi: 10.1139/m88-067. [DOI] [PubMed] [Google Scholar]
- 4.Beveridge T J. Bacterial S-layers. Curr Opin Struct Biol. 1994;4:204–212. [Google Scholar]
- 5.Beveridge T J, Koval S F, editors. Advances in paracrystalline surface layers. New York, N.Y: Plenum Press; 1993. [Google Scholar]
- 6.Beveridge T J, Makin S A, Kadurugamuwa J L, Li Z. Interactions between biofilms and the environment. FEMS Microbiol Rev. 1997;20:291–303. doi: 10.1111/j.1574-6976.1997.tb00315.x. [DOI] [PubMed] [Google Scholar]
- 7.Beveridge T J, Popkin T J, Cole R M. Electron microscopy. In: Gerhardt P, editor. Methods for general molecular bacteriology. Washington, D.C: American Society for Microbiology; 1994. pp. 42–71. [Google Scholar]
- 8.Etienne-Toumelin I, Sirard J-C, Duflot E, Mock M, Fouet A. Characterization of Bacillus anthracis S-layer: cloning and sequencing of the structural gene. J Bacteriol. 1995;177:614–620. doi: 10.1128/jb.177.3.614-620.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Gilmore R D, Joste J N, McDonald G A. Cloning, expression and sequence analysis of the gene encoding the 120 kD surface-exposed protein of Rickettsia rickettsii. Mol Microbiol. 1989;3:1579–1586. doi: 10.1111/j.1365-2958.1989.tb00143.x. [DOI] [PubMed] [Google Scholar]
- 10.Graham L L, Beveridge T J. Evaluation of freeze-substitution and conventional protocols for routine electron microscopic processing of eubacteria. J Bacteriol. 1990;172:2141–2149. doi: 10.1128/jb.172.4.2141-2149.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Graham L L, Beveridge T J. Effect of chemical fixatives on accurate preservation of Escherichia coli and Bacillus subtilis structure in cells prepared by freeze-substitution. J Bacteriol. 1990;172:2150–2159. doi: 10.1128/jb.172.4.2150-2159.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Gruber K, Sleytr U B. Localized insertion of new S-layer during growth of Bacillus stearothermophilus strains. Arch Microbiol. 1988;149:485–491. doi: 10.1007/BF00446749. [DOI] [PubMed] [Google Scholar]
- 13.Ishiguro E E, Kay W W, Ainsworth T, Buckley J T, Trust T J. Loss of virulence during culture of Aeromonas salmonicida at high temperature. J Bacteriol. 1981;148:333–340. doi: 10.1128/jb.148.1.333-340.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kadurugamuwa J L, Beveridge T J. Virulence factors are released from Pseudomonas aeruginosa in association with membrane vesicles during normal growth and exposure to gentamicin: a novel mechanism of enzyme secretion. J Bacteriol. 1995;177:3998–4008. doi: 10.1128/jb.177.14.3998-4008.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kadurugamuwa J L, Beveridge T J. The bacteriolytic effect of membrane vesicles from Pseudomonas aeruginosa on other bacteria including pathogens: conceptually new antibiotics. J Bacteriol. 1996;178:2767–2774. doi: 10.1128/jb.178.10.2767-2774.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15a.Kadurugamuwa, J. L., and T. J. Beveridge. Unpublished data.
- 16.Koval S F, Murray R G E. Isolation of surface array proteins from bacteria. Can J Biochem Cell Biol. 1984;62:1181–1189. doi: 10.1139/o84-152. [DOI] [PubMed] [Google Scholar]
- 17.Li Z, Clarke A J, Beveridge T J. A major autolysin of P. aeruginosa, its subcellular distribution, its potential role in cell growth and division, and its secretion in surface membrane vesicles. J Bacteriol. 1996;178:2479–2488. doi: 10.1128/jb.178.9.2479-2488.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Meier-Stauffer K, Busse H-J, Rainey F A, Burghardt J, Scheberl A, Hollaus F, Kuen B, Makristathis A, Sleytr U B, Messner P. Description of Bacillus thermoaerophilus sp. nov., to include sugar beet isolates and Bacillus brevis ATCC 12990. Int J Syst Bacteriol. 1996;46:532–541. [Google Scholar]
- 19.Mesnage S, Tosi-Couture E, Mock M, Gounon P, Fouet A. Molecular characterization of the Bacillus anthracis main S-layer component: evidence that it is the major cell-associated antigen. Mol Microbiol. 1997;23:1147–1155. doi: 10.1046/j.1365-2958.1997.2941659.x. [DOI] [PubMed] [Google Scholar]
- 20.Messner P. Bacterial glycoproteins. Glycoconj J. 1997;14:3–11. doi: 10.1023/a:1018551228663. [DOI] [PubMed] [Google Scholar]
- 21.Messner P, Sleytr U B. Separation and purification of S-layers from gram-positive and gram-negative bacteria. In: Hancock I C, Poxton I R, editors. Bacterial cell surface techniques. Chichester, United Kingdom: John Wiley & Sons; 1988. pp. 97–104. [Google Scholar]
- 22.Messner P, Sleytr U B. Bacterial surface layer (S-layer) glycoproteins. Glycobiology. 1991;1:545–551. doi: 10.1093/glycob/1.6.545. [DOI] [PubMed] [Google Scholar]
- 23.Messner P, Sleytr U B. Crystalline bacterial cell surface layers. Adv Microb Physiol. 1992;33:213–275. doi: 10.1016/s0065-2911(08)60218-0. [DOI] [PubMed] [Google Scholar]
- 24.Messner P, Hollaus F, Sleytr U B. Paracrystalline cell wall surface layers of different Bacillus stearothermophilus strains. Int J Syst Bacteriol. 1984;34:202–210. [Google Scholar]
- 25.Pei Z, Blaser M J. Pathogenesis of Campylobacter infections. Role of surface array proteins in virulence in a mouse model. J Clin Invest. 1990;85:1036–1043. doi: 10.1172/JCI114533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Sára M, Kuen B, Mayer H F, Mandl F, Schuster K C, Sleytr U B. Dynamics in oxygen-induced changes in S-layer protein synthesis from Bacillus stearothermophilus PV72 and the S-layer-deficient variant T5 in continuous culture and studies of cell wall composition. J Bacteriol. 1996;178:2108–2117. doi: 10.1128/jb.178.7.2108-2117.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Sára M, Sleytr U B. Molecular sieving through S layers of Bacillus stearothermophilus strains. J Bacteriol. 1987;169:4092–4098. doi: 10.1128/jb.169.9.4092-4098.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Sleytr U B, Messner P, Pum D, Sára M, editors. Crystalline bacterial cell surface layers. Berlin, Germany: Springer-Verlag; 1988. [Google Scholar]
- 29.Sleytr U B, Messner P, Pum D, Sára M. Crystalline bacterial cell surface layers. Mol Microbiol. 1993;10:911–916. doi: 10.1111/j.1365-2958.1993.tb00962.x. [DOI] [PubMed] [Google Scholar]
- 30.Sleytr U B, Messner P, Pum D, Sára M, editors. Crystalline bacterial cell surface proteins. R.G. Austin, Tex: Landes Co./Academic Press Inc.; 1996. [Google Scholar]
- 31.Sleytr U B, Thornley M J. Freeze-etching of the cell envelope of an Acinetobacter species which carries a regular array of surface subunits. J Bacteriol. 1973;116:1383–1397. doi: 10.1128/jb.116.3.1383-1397.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Sprott G D, Koval S F, Schnaitman C A. Cell fractionation. In: Gerhardt P, editor. Methods for general molecular bacteriology. Washington, D.C: American Society for Microbiology; 1994. pp. 72–103. [Google Scholar]
- 33.Work E. Reaction of ninhydrin in acid solution with straight-chain amino acids containing two amino groups and its application to the estimation of αɛ-diaminopimelic acid. Biochem J. 1957;67:4216–4230. doi: 10.1042/bj0670416. [DOI] [PMC free article] [PubMed] [Google Scholar]