Abstract
Restraint water-immersion stress (RWIS) can induce anxiety, hypothermia, and severe vagally-mediated gastric dysfunction. The present work explored the effects of different durations of RWIS on neuronal activities of the forebrain by c-Fos expression in conscious rats exposed to RWIS for 0, 30, 60, 120, or 180 min. The peak of c-Fos induction was distinct for different forebrain regions. The most intense c-Fos induction was always observed in the supraoptic nucleus (SON), and then in the hypothalamic paraventricular nucleus (PVN), posterior cortical amygdaloid nucleus (PCoA), central amygdaloid nucleus (CeA), and medial prefrontal cortex (mPFC). Moreover, body temperature was reduced to the lowest degree after 60 min of RWIS, and the gastric lesions tended to gradually worsen with the prolonging of RWIS duration. These data strongly suggest that these nuclei participate in the organismal response to RWIS to different degrees, and may be involved in the hypothermia and gastric lesions induced by RWIS.
Keywords: c-Fos expression, Supraoptic nucleus, Restraint water-immersion stress, Gastric lesions, Hypothermia
Introduction
Restraint water-immersion stress (RWIS), considered to be a mixture of physical and psychological stressors, can induce anxiety, hypothermia, and severe gastric dysfunction including gastric hypercontractility, gastric acid hypersecretion, and gastric mucosal lesions within a few hours [1–4]. The gastric lesions induced by RWIS can be eliminated, however, if the rats are pretreated with papaverine HCl [5], pentobarbital sodium [3], bilateral subdiaphragmatic vagotomy [1], or atropine [3]. These results strongly suggest that the stress gastric lesions were mainly due to neuronal activities in the brain and mediated by the vagal parasympathetic efferent pathway.
c-Fos protein, a nuclear phosphoprotein product of the immediate early gene c-fos, has been extensively used as a marker of neuronal activation [6]. Mapping studies showed that acute stress could induce the expression of c-Fos rapidly and transiently in specific brain regions, including the hypothalamic paraventricular nucleus (PVN), lateral septum (LS), amygdala, cerebral cortex, and dorsal vagal complex (DVC) as well [7–9]. The induction of brain c-Fos expression during acute stress depends on the intrinsic nature, intensity, and duration of the stressor [10–12]. To date, studies on the temporal–spatial pattern of c-Fos expression induced by stress mainly focus on the acute restraint stress, which is a mild psychological stressor and does not induce gastric lesions and quick hypothermia in a short time.
Only two studies have investigated neuronal activation in response to RWIS [13, 14]. Maruyama et al. [13] reported that RWIS dramatically increased c-Fos protein accumulation in the nuclei of medulla A1/A2 noradrenergic neurons and dorsomedial hypothalamic nucleus (DMH) prolactin-releasing peptide (PrRP) neurons. Our previous study showed RWIS could induce remarkable c-Fos expression in the DVC and nucleus ambiguus (NA), which are the primary nerve centers for regulating gastric functions, but the most intense c-Fos expression was observed in the dorsal motor nucleus of the vagus (DMV) and NA [14]. However, which regions of the forebrain are activated by RWIS and the temporal–spatial pattern of neuronal activities under RWIS have not been reported so far. Moreover, the forebrain is the higher brain center regulating visceral functions and is responsible for forming emotional reactions to the stress. In this paper, the effects of different RWIS durations on neuronal activities of the forebrain were investigated with c-Fos expression as a marker of neuronal activation.
Materials and methods
Experimental animals
Male Wistar rats (Experimental Animal Center of Shandong University, Jinan, China), weighing 170–200 g, were individually housed in cages for at least 7 days at an ambient temperature of 22 ± 2°C with a normal day/night cycle before the experiments. The animals had ad libitum access to pelleted food and tap water. Before stress, the rats were fasted for 24 h, but allowed free access to water. All stresses were finished between 0800 and 1200 h to minimize circadian rhythm-related variations in the stress response. All procedures were performed in accordance with the Japanese Physiological Society’s Guiding Principles for the Care and Use of Animals in the Field of Physiological Sciences.
Grouping and stress protocols
The rats were randomly divided into five groups designated according to their durations of RWIS: 0, 30, 60, 120, and 180, with five rats in each group. Under light ether anesthesia, the four limbs of each rat in groups 30, 60, 120, and 180 were bound on a wooden board gently but securely using medical adhesive tape. After the rats were conscious, they were vertically immersed in cold water (21 ± 1°C) to the level of the xiphoid for 30, 60, 120, or 180 min. Rats in group 0 (control group) were not stressed under otherwise identical conditions. At the end of the procedure, the rats were deeply anesthetized with an overdose of pentobarbital sodium (100 mg/kg body weight, i.p.).
Additionally, five rats in another group (group 30-30) were given RWIS for 30 min and then replaced in their home cages for 30 min before sacrifice. Group 30-30 was used for comparison with group 30 and group 60 to determine the intrinsic kinetics of c-Fos expression.
Evaluation of body temperature and gastric lesions
At the end of the stress experiment, the abdomen of the anesthetized rat was opened by a small midline incision, then the celiac temperature was measured and the stomach was removed and fixed with 1% formalin. The gastric lesions were examined with a hand magnifying lens, and the erosion index (EI) was evaluated by the score system [15]. Scores were given according to the length of lesions: ≤1 mm = 1 point, 1 to ≤2 mm = 2 point, and so on. The score was multiplied by 2 when the lesion was more than 1 mm in width. The cumulative scores of all lesions in a rat served as EI of the rat.
Immunohistochemistry
The rats were perfused transcardially with 0.01 M phosphate-buffered saline (PBS, pH 7.4) followed by 500 ml freshly prepared 4% paraformaldehyde in 0.1 M phosphate buffer (4°C). Afterwards, the forebrains were removed immediately, post-fixed in the same fixative for 4 h, and cryoprotected overnight in 20% sucrose in 0.1 M PBS at 4°C until sectioning. Series of 40-μm coronal sections of the forebrain at the level of the frontal lobe and the hypothalamus were then cut with a freezing microtome and collected into 0.01 M PBS. The free-floating sections (a 1-in-4 series of the sections taken from each animal) were pretreated for 30 min in methanolic 3% H2O2 to eliminate endogenous peroxidase activity. After being rinsed in 0.01 M PBS, they were incubated with blocking buffer (5% normal goat serum and 0.3% Triton X-100 in PBS) for 30 min, then with rabbit anti-c-Fos antibody (sc-52, Santa Cruz Biotechnology, Santa Cruz, CA, USA) at a dilution of 1:2,000 for 24 h at 4°C. Subsequently, the sections were incubated with the biotinylated goat anti-rabbit IgG (Zymed Laboratories, San Fransisco, CA, USA) for 1 h at room temperature and then with streptavidin-biotin-horseradish peroxidase complex (Zymed) for 1 h at room temperature. The sections were submitted to a diaminobenzidine reaction, yielding a brown nuclear deposit. Between steps, the sections were rinsed completely in PBS containing 1% Triton X-100 (PBST). Sections were mounted on gelatin-coated glass slides, restained with hematoxylin, dehydrated in a series of alcohols, cleared in xylene, and coverslipped. The specificity of the immunostaining was verified by incubation of the brain sections with normal goat serum, which produced no staining.
Counting c-Fos-positive neurons
Pictures of brain sections were taken under identical conditions with a BX51 Olympus microscope (Olympus, Tokyo, Japan) coupled to an Olympus DP70 camera. The nomenclature and nuclear boundaries defined in the rat brain stereotaxic atlas of Paxinos and Watson [16] were used in this study. c-Fos-positive nuclear profiles in different areas of the forebrain were counted using Image-Pro Plus 6.0 (Media Cybernetics, Silver Spring, MD, USA). The number and integrated optical density (IOD) of c-Fos-positive neurons within each area were counted bilaterally (where possible) in consecutive sections per animal. The average values in 0.01 mm2 are reported as number and IOD of c-Fos-positive neurons, which denote the intensity of c-Fos expression.
Statistical analysis
All data on time course are presented as means ± SEM. With SPSS13.0 software (SPSS, Chicago, IL, USA), statistical analyses regarding time course data (groups 0, 30, 60, 120, 180) were performed by one-way or two-way analysis of variance (ANOVA) followed by Tukey’s post-hoc test individually. Statistical comparisons between two groups (group 30-30 vs. group 30 and group 30-30 vs. group 60) were performed by two-tailed independent sample t tests. The level of significance was set at P < 0.05.
Additionally, in order to determine the reactive extent of some nuclei in the forebrain in response to the stress, the multiples of controls were counted [17], that is, the quantum of c-Fos expression of each rat was divided by the mean values of the control group. Then, “average multiples of controls” = (the multiples of control number + the multiples of control IOD of c-Fos-positive neurons)/2. The average multiples were used as the relative intensity of c-Fos expression because they could more accurately reflect the differences in c-Fos expression among the different nuclei in response to stress.
Results
Effects of different RWIS durations on c-Fos expression in the forebrain
Neuronal activation was assessed on the basis of c-Fos-immunoreactivity, a brown deposit in the cell nucleus. The number and IOD of c-Fos-positive neurons in the forebrain in rats under continuous stress are shown in Table 1. In the control group (group 0), there were very few c-Fos-positive neurons in the forebrain. In the four groups under continuous stress (groups 30, 60, 120, and 180), however, many remarkable c-Fos-positive neurons were observed in the forebrain, especially in the supraoptic nucleus (SON), PVN, suprachiasmatic nucleus (SCh), and ventrolateral septum (LSv). Two-way ANOVAs of the number of c-Fos-positive neurons revealed significant overall effects of duration of stress (P < 0.001), regional response (P < 0.001), and their combined interaction (P < 0.001). Similar effects could also be achieved by two-way ANOVAs of the IOD of c-Fos-positive neurons.
Table 1.
The number and IOD of c-Fos-positive neurons in the forebrain in rats under continuous stress (n = 5)
| Group 0 | Group 30 | Group 60 | Group 120 | Group 180 | |
|---|---|---|---|---|---|
| Midline cortical areas | |||||
| mPFC | 0.95 ± 0.20a | 2.36 ± 0.26b | 4.01 ± 0.75c | 3.50 ± 0.39bc | 2.60 ± 0.17b |
| 2.30 ± 0.52a | 7.17 ± 0.91ab | 17.56 ± 4.03c | 12.53 ± 2.43bc | 8.72 ± 0.97ab | |
| Cg | 0.90 ± 0.16a | 1.55 ± 0.28ab | 2.71 ± 0.60c | 2.16 ± 0.24bc | 1.41 ± 0.10ab |
| 2.56 ± 0.59a | 4.50 ± 0.97a | 9.37 ± 2.51b | 6.35 ± 0.88ab | 3.87 ± 0.29a | |
| RS | 0.89 ± 0.20a | 1.62 ± 0.31ab | 2.36 ± 0.53b | 1.37 ± 0.25a | 1.23 ± 0.13a |
| 2.06 ± 0.54a | 3.48 ± 0.76a | 6.83 ± 1.57b | 3.25 ± 0.54a | 2.83 ± 0.31a | |
| Septal area | |||||
| LSv | 2.63 ± 0.22a | 5.69 ± 0.77b | 6.25 ± 0.43b | 8.17 ± 0.32c | 7.57 ± 0.30c |
| 7.97 ± 0.81a | 21.05 ± 4.22b | 31.24 ± 3.62c | 40.99 ± 4.31c | 37.81 ± 1.52c | |
| Amygdala | |||||
| CeA | 0.71 ± 0.15a | 2.18 ± 0.27b | 2.80 ± 0.33b | 4.96 ± 0.31c | 4.35 ± 0.73c |
| 2.70 ± 0.72a | 5.94 ± 0.78a | 11.36 ± 2.02ab | 23.73 ± 2.51c | 19.28 ± 4.68bc | |
| MeA | 0.92 ± 0.21a | 2.32 ± 0.02b | 2.37 ± 0.45b | 3.32 ± 0.35c | 2.74 ± 0.30bc |
| 3.85 ± 1.06a | 9.95 ± 0.77b | 13.06 ± 3.03bc | 18.65 ± 2.65c | 13.49 ± 1.81bc | |
| PCoA | 0.46 ± 0.07a | 1.97 ± 0.19b | 2.58 ± 0.26bc | 3.07 ± 0.47c | 2.52 ± 0.14bc |
| 3.02 ± 0.74a | 10.40 ± 0.65b | 18.00 ± 2.65c | 22.01 ± 3.92c | 16.25 ± 0.93bc | |
| Hypothalamus | |||||
| MPO | 1.14 ± 0.24a | 2.09 ± 0.25b | 1.97 ± 0.50ab | 2.87 ± 0.30b | 2.08 ± 0.10b |
| 3.27 ± 0.84a | 6.86 ± 0.78ab | 7.35 ± 2.52ab | 10.78 ± 1.27b | 6.22 ± 0.47a | |
| AH | 1.51 ± 0.34a | 2.62 ± 0.20c | 2.39 ± 0.23bc | 2.18 ± 0.07bc | 1.81 ± 0.14ab |
| 5.32 ± 1.41a | 9.92 ± 0.79bc | 11.12 ± 1.67c | 7.77 ± 0.55ab | 5.71 ± 0.29a | |
| PVN | 1.47 ± 0.32a | 6.70 ± 0.89b | 8.50 ± 0.37b | 7.26 ± 1.10b | 7.89 ± 0.92b |
| 2.59 ± 0.63a | 17.06 ± 1.98b | 27.70 ± 2.87b | 23.87 ± 4.47b | 28.91 ± 5.34b | |
| SON | 0.54 ± 0.10a | 4.67 ± 0.87b | 7.65 ± 1.43b | 7.43 ± 1.47b | 5.82 ± 1.05b |
| 1.30 ± 0.29a | 18.05 ± 5.17ab | 43.60 ± 9.45c | 37.82 ± 10.07bc | 26.59 ± 7.24bc | |
| SCh | 1.13 ± 0.25a | 7.89 ± 0.48c | 6.14 ± 1.31bc | 8.43 ± 1.01c | 4.55 ± 0.62b |
| 3.00 ± 0.78a | 22.59 ± 1.63bc | 20.54 ± 5.59b | 31.90 ± 3.91c | 10.70 ± 1.79a | |
| DMH | 2.08 ± 0.47a | 3.31 ± 0.34b | 3.47 ± 0.42b | 4.37 ± 0.40b | 3.99 ± 0.14b |
| 6.19 ± 1.66a | 13.16 ± 2.58ab | 16.98 ± 3.06b | 19.91 ± 3.44b | 15.19 ± 0.39b | |
| Arc | 1.74 ± 0.43a | 3.28 ± 0.37ab | 4.02 ± 0.46b | 3.84 ± 0.71b | 3.71 ± 0.60b |
| 5.22 ± 1.44a | 11.73 ± 1.25ab | 18.36 ± 2.65b | 17.88 ± 3.90b | 15.28 ± 3.10b | |
| SuM | 1.84 ± 0.41a | 2.96 ± 0.13b | 3.47 ± 0.36bc | 4.48 ± 0.22c | 3.64 ± 0.38bc |
| 6.96 ± 1.78a | 13.91 ± 0.94b | 20.55 ± 3.11bc | 22.04 ± 1.78c | 15.98 ± 1.32bc | |
For each nucleus, the data in the top row represent the number of c-Fos-positive neurons, and the data in the bottom row represent the IOD of c-Fos-positive neurons
Means in a row without a common letter represent significant difference at P < 0.05
AH Anterior hypothalamic area, Arc arcuate hypothalamic nucleus, CeA central amygdaloid nucleus, Cg cingulated cortex, DMH dorsomedial hypothalamic nucleus, LSv ventrolateral septum, MeA medial amygdaloid nucleus, mPFC medial prefrontal cortex, MPO medial preoptic nucleus, PCoA posterior cortical amygdaloid nucleus, PVN hypothalamic paraventricular nucleus, RS retrosplenial cortex, SCh suprachiasmatic nucleus, SON supraoptic nucleus, SuM supramammillary nucleus
One-way ANOVA indicated a significant effect of duration of RWIS on c-Fos expression for each observed nucleus of the forebrain (P < 0.05). Post-hoc tests showed that c-Fos expression in most nuclei of the hypothalamus, especially the SON and PVN, was significantly greater in rats stressed for 30 min than in controls and then remained at a higher level (Figs. 1 and 2; Table 1). c-Fos expression in the midline cortical areas, including the medial prefrontal cortex (mPFC), cingulated cortex (Cg), and retrosplenial cortex (RS), peaked at 60 min of stress, and subsequently decreased gradually (Table 1). c-Fos expression in the LSv and amygdala, including the central amygdaloid nucleus (CeA), medial amygdaloid nucleus (MeA), and posterior cortical amygdaloid nucleus (PCoA), tended to peak at 120 min of stress (Table 1).
Fig. 1.
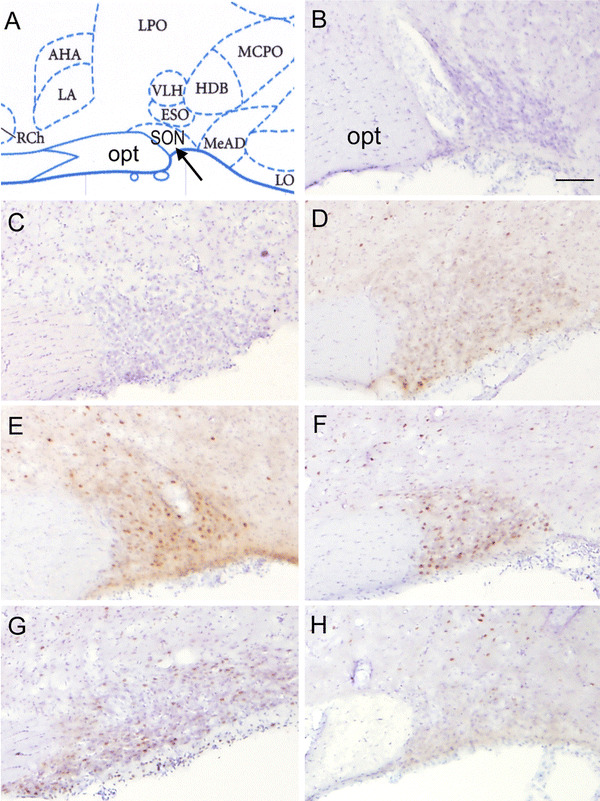
Representative sections showing c-Fos expression in the supraoptic nucleus (SON) induced by different RWIS durations. a The exact location of the SON. b The specificity of the immunostaining verified by incubation of the brain sections of group 60 rats with normal goat serum. c There were very few c-Fos-positive neurons in the SON in group 0, but there were many prominent c-Fos-positive neurons in the SON in groups 30 (d), 60 (e), 120 (f), and 180 (g), and the expression of c-Fos tended to be maintained at a higher level during the stress. h There were few c-Fos-positive neurons in the SON in group 30-30. In the whole panel, brownish oval or round spots are the cellular nuclei of the c-Fos-positive neurons, and blue spots are the cellular nuclei of the c-Fos-negative neurons. Scale bars 100 μm. opt Optic tract
Fig. 2.
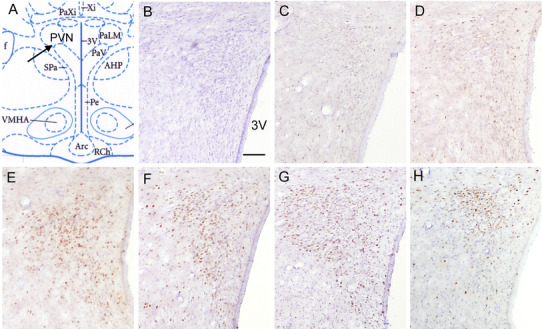
Representative sections showing c-Fos expression in the hypothalamic paraventricular nucleus (PVN) induced by different RWIS durations. a The exact location of the PVN. b The specificity of the immunostaining verified by incubation of the brain sections of group 60 rats with normal goat serum. In the control group, there were very few c-Fos-positive neurons in the PVN (c), but there were many remarkable c-Fos-positive neurons in the PVN in groups 30 (d), 60 (e), 120 (f), and 180 (g). h There were fewer c-Fos-positive neurons in group 30-30 than group 60. Scale bars 100 μm. 3V Third ventricle
Comparison of c-Fos expression induced by RWIS in different nuclei of the forebrain
As the basal level of c-Fos expression in different nuclei was very diverse in the control group (Table 1), the relative intensity of c-Fos expression (Table 2) could more generally reflect the differences in c-Fos expression among the different nuclei. Nuclei with a relative intensity of c-Fos expression that was more than fourfold the controls were selected, then ranked from high to low intensity as the SON, SCh, and PVN in group 30; the SON, PVN, SCh, mPFC, PCoA, and CeA in group 60; the SON, SCh, CeA, PVN, PCoA, mPFC, MeA, and LSv in group 120; the SON, PVN, CeA, and PCoA in group 180. Therefore, a striking new finding in the present study is that the most intense expression of c-Fos was always observed in the SON in the four groups under continuous stress, followed by the PVN, SCh, PCoA, CeA, mPFC, MeA, and LSv.
Table 2.
The relative intensity of c-Fos expression in the forebrain in rats under continuous stress (average multiples of controls)
| Group 0 | Group 30 | Group 60 | Group 120 | Group 180 | |
|---|---|---|---|---|---|
| Midline cortical areas | |||||
| mPFC | 1 | 2.80 | 5.93 | 4.57 | 3.26 |
| Cg | 1 | 1.74 | 3.34 | 2.44 | 1.54 |
| RS | 1 | 1.75 | 2.98 | 1.56 | 1.38 |
| Septal area | |||||
| LSV | 1 | 2.40 | 3.15 | 4.12 | 3.81 |
| Amygdala | |||||
| CeA | 1 | 2.64 | 4.08 | 7.89 | 6.63 |
| MeA | 1 | 2.55 | 2.98 | 4.23 | 3.24 |
| PCoA | 1 | 3.86 | 5.78 | 6.98 | 5.43 |
| Hypothalamus | |||||
| MPO | 1 | 1.97 | 1.99 | 2.91 | 1.87 |
| AH | 1 | 1.80 | 1.83 | 1.45 | 1.13 |
| PVN | 1 | 5.57 | 8.24 | 7.08 | 8.27 |
| SON | 1 | 11.27 | 23.83 | 21.42 | 15.60 |
| SCh | 1 | 7.28 | 6.16 | 9.07 | 3.81 |
| DMH | 1 | 1.86 | 2.21 | 2.66 | 2.18 |
| Arc | 1 | 2.07 | 2.92 | 2.82 | 2.53 |
| SuM | 1 | 1.80 | 2.42 | 2.80 | 2.14 |
Average multiples of controls = (the multiples of control number + the multiples of control IOD of c-Fos-positive neurons)/2
Comparison of c-Fos expression in some regions of the forebrain between group 30-30 and groups 30 and 60
The expression of c-Fos in the eight aforementioned nuclei of rats stressed for 30 min and given a 30-min recovery period (group 30-30) is shown in Table 3. The expression of c-Fos in the SON (Fig. 1h) and CeA of the rats in group 30-30 was significantly less than that in group 30 (P < 0.05), while the c-Fos expression in the other nuclei (except LSv) in group 30-30 tended to be smaller than that in group 30, although no significant difference in c-Fos expression (P > 0.05) could be observed. The notable decrease in the c-Fos expression in the SON and CeA induced by withdrawal of RWIS implied that the neurons in the SON and CeA were particularly sensitive to RWIS.
Table 3.
The expression of c-Fos in some nuclei of the forebrain in rats in group 30-30, group 30 and group 60 (n or IOD/0.01 mm2) (n = 5)
| Group 30 (n) | Group 30-30 (n) | Group 60 (n) | Group 30 (IOD) | Group 30-30 (IOD) | Group 60 (IOD) | |
|---|---|---|---|---|---|---|
| mPFC | 2.36 ± 0.26 | 1.88 ± 0.11 | 4.01 ± 0.75* | 7.17 ± 0.91 | 5.56 ± 0.68 | 17.56 ± 4.03* |
| LSv | 5.69 ± 0.77 | 7.20 ± 0.15 | 6.25 ± 0.43 | 21.05 ± 4.22 | 27.18 ± 2.17 | 31.24 ± 3.62 |
| CeA | 2.18 ± 0.27* | 1.02 ± 0.10 | 2.80 ± 0.33* | 5.94 ± 0.78* | 2.33 ± 0.23 | 11.36 ± 2.02* |
| MeA | 2.32 ± 0.02 | 1.80 ± 0.31 | 2.37 ± 0.45 | 9.95 ± 0.77 | 6.16 ± 1.75 | 13.06 ± 3.03 |
| PCoA | 1.97 ± 0.19 | 1.71 ± 0.29 | 2.58 ± 0.26* | 10.40 ± 0.65 | 7.43 ± 1.76 | 18.00 ± 2.65* |
| PVN | 6.70 ± 0.89 | 4.95 ± 0.51 | 8.50 ± 0.37* | 17.06 ± 1.98 | 12.78 ± 1.83 | 27.70 ± 2.87* |
| SON | 4.67 ± 0.87* | 0.44 ± 0.05 | 7.65 ± 1.43* | 18.05 ± 5.17* | 1.38 ± 0.32 | 43.60 ± 9.45* |
| SCh | 7.89 ± 0.48 | 5.92 ± 1.90 | 6.14 ± 1.31 | 22.59 ± 1.63 | 19.53 ± 5.92 | 20.54 ± 5.59 |
* P < 0.05 compared with group 30-30 for each nucleus
The expression of c-Fos in the SON (Fig. 1h), CeA, PVN (Fig. 2h), PCoA, and mPFC of the rats in group 30-30 was significantly less than that in group 60 (P < 0.05); and the c-Fos expression in the SCh, LSv, and MeA was not significantly different between the two groups. This could indicate more c-Fos expression in these five nuclei of rats given 60 min of RWIS than in those of rats given 30 min of RWIS.
The changes in body temperature induced by different RWIS durations
RWIS induced prominent hypothermia in rats (Fig. 3). One-way ANOVA showed a significant effect of RWIS duration on the body temperature (P < 0.001). Post-hoc tests indicated that body temperature was significantly lower in rats stressed for 30 min than in controls, and even lower in rats stressed for 60 min, whereafter it remained at a lower level. Rats in group 30-30 had significantly higher body temperature than rats in group 30 and group 60 (P < 0.001).
Fig. 3.
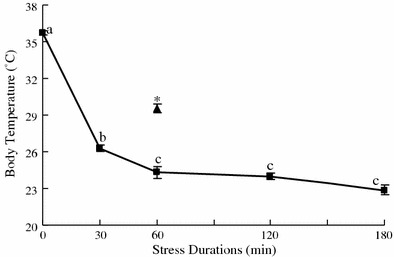
Hypothermia induced by RWIS. Body temperature was measured in the rats at 0, 30, 60, 120, or 180 min of RWIS (squares). An additional data point represents body temperature of rats given 30 min of RWIS followed by 30 min of recovery (triangle). Data are presented as means ± SEM (n = 5). Data without a common letter represent significant difference at P < 0.05 (Tukey’s post-hoc test). Asterisk represents a significant difference at P < 0.05 compared with group 60
Gastric lesions induced by different RWIS durations
No macroscopic gastric lesions could be observed in the control group, but in the four groups under continuous stress, gastric lesions tended to gradually worsen with increasing durations of RWIS (P < 0.05). The erosion indices of groups 30, 60, 120, and 180 were 6.0 ± 1.3, 11.8 ± 1.9, 20.0 ± 2.2, and 27.5 ± 3.1, respectively. The EI of group 30-30 was 7.75 ± 1.68, slightly less than that of group 60.
Discussion
The present results show that RWIS induced c-Fos expression in a restricted population of neurons in the forebrain, especially in the hypothalamus, for there were no c-Fos-positive cells or a few scattered immunoreactive cells in the same brain areas in non-stressed rats. The absence of c-Fos-positive cells or the presence of a few scattered cells in brain neurons is representative of non-stressed basal conditions [18]. Moreover, the intensity of c-Fos expression within specific forebrain regions was dissimilar for different RWIS durations, suggesting diverse increases in neuronal activity within these areas induced by RWIS.
Within the different RWIS sessions, robust c-Fos expression was induced in the hypothalamus, which indicated that a lot of neurons in the hypothalamus were excited during RWIS. This is consistent with the idea that the hypothalamus plays important roles in the autonomic nervous responses to stress. Interestingly, the present study found the most intense c-Fos expression was always observed in the SON and then in the PVN and SCh during RWIS. A previous study found different durations (0.5, 1, 2, and 4 h stress) of restraint stress (not inducing the gastric lesions and hypothermia) also induced marked increase in c-Fos expression in the PVN [10]. However, immobilization stress (taping each rat’s limbs to a metal frame with the rat kept in a prone position) for 30 min induced c-Fos expression in neurons of the dorsal part of the SON, most likely oxytocin-containing cells, while immobilization stress for 2–3 h induced c-Fos expression in the entire SON [19]. In our experiment, RWIS always induced robust c-Fos expression in the entire SON within 3 h. The distinctions of c-Fos expression in the SON may be due to the nature of the stressors among studies. The SON, PVN, and SCh are all located in the anterior part of the hypothalamus, which is considered to be the center of the parasympathetic nervous system. Our previous study found RWIS could also induce activation of many neurons in the DMV and NA, which are the final efferent pathways of the central parasympathetic system modulating visceral activity [14]. Moreover, RWIS induced a low level of c-Fos expression in the DMH, arcuate hypothalamic nucleus (Arc), and supramammillary nucleus (SuM). The DMH, Arc, and SuM are located in the posterior part of the hypothalamus, which is the center of the sympathetic nervous system. All these findings suggest that RWIS mainly induces the strong activation of the parasympathetic nervous system and induces a slight activation of the sympathetic nervous system.
In the present experiment, RWIS resulted in a marked decrease in body temperature and the formation of acute gastric lesions. Rapid hypothermia during RWIS was assumed to participate somewhere in the induction of a definite parasympathetic predominance because restraint alone, not following a marked fall in body temperature, did not induce the parasympathetic dominance [2, 3, 20, 21]. Furthermore, gastric lesions induced by RWIS resulted from parasympathetic overactivity [3, 4, 21, 22]. Thus, the anterior part of the hypothalamus must be implicated in regulating the gastric pathologies induced by RWIS, which can be supported by many previous studies. Zhang et al. [23] reported electric stimulation of PVN and microinjection of l-glutamate into PVN could obviously increase the gastric mucosal lesion induced by RWIS, while PVN lesion could significantly decrease it; and these effects were attenuated by vagotomy or atropine injected subcutaneusly. Eugene and Koplovitz [24] indicated microinjection of pilocarpine hydrochloride and methacholine chloride into the SON produced significant increases in gastric secretory volume, titratable acid, and chloride and pepsin output in dogs, which was abolished by bilateral vagotomy. The SCh mediates the central circadian clock oscillation, which has an important role in the formation of stress-induced gastric lesions [25, 26].
The amygdala is a heterogeneous brain structure [27, 28] and has direct fibrous connections with the hypothalamus [29, 30] and DVC [31]. RWIS induced prominent c-Fos expression in the CeA, MeA, and PCoA, and the peak of c-Fos expression in these divisions occured at 120 min of stress, which further proved the amygdala was a critical structure for the maintenance of gastric mucosal integrity [32, 33]. Electrical stimulation of either the CeA or MeA induced gastric erosions, whereas this effect could be eliminated by prior lesions of the ventral amygdalofugal pathway, bilateral cervical vagotomy, or acute atropine pretreatments [34, 35]. Lesions in the dorsomedial amygdala (CeA and dorsal part of MeA) could attenuate gastric pathology induced by experimental stressors, but lesions in the posterolateral amygdala (PCoA, posterior part of basolateral amygdaloid nucleus, and entorhinal cortex) had opposite effects [36].
In the midline cortical areas, the expression of c-Fos reached a maximum at 60 min of stress, which showed the activities of neurons in the areas were strongest then, probably because the areas participated in informational processes and cognitive evaluation in the earlier stress response [37]. Henke [38] pointed out that the mPFC are inhibitory structures, because lesions in the mPFC apparently aggravated gastric pathologies induced by restraint stress for 24 h, which demonstrated the importance of mPFC in gastric pathologies induced by stress.
However, rats stressed for 30 min followed by 30 min of recovery had less c-Fos expression in the SON and CeA than rats only stressed for 30 min, which indicated the neurons in the SON and CeA are sensitive to the recovery following the stress. Rats stressed continuously for 60 min had more c-Fos expression in the SON, PVN, PCoA, CeA, and mPFC than rats stressed for 30 min followed by 30 min of recovery, which could somewhat reflect more neuronal activation in these regions of rats given 60 min of RWIS than rats given 30 min of RWIS. This suggests that the c-Fos expression in the above nuclei was indubitably induced by RWIS and sensitive to stressor duration [10, 14]. Thus, the pattern of neuronal activities in the SON, PVN, PCoA, CeA, and mPFC depends on stressor duration. As recovery for 30 min after RWIS induced a significant increase in lower body temperature and did not obviously aggravate the gastric lesions induced by RWIS, the low level of c-Fos expression in rats stressed for 30 min followed by 30 min of recovery further proved the important role of the five above nuclei in regulating the gastric pathologies and hypothermia induced by RWIS.
In summary, RWIS induced the most intense c-Fos expression in the SON and intense expression in the PVN, PCoA, CeA, and mPFC; the intensity of c-Fos expression in these nuclei depended on the duration of RWIS. The peaks of the activities of these nuclei were distinct during RWIS. The increased neuronal activities in those nuclei may be involved in modulating the organismal responses to RWIS and the hypothermia and gastric lesions induced by RWIS. The present study, together with our previous study, provides a neuroanatomical evidence that RWIS induces hyperactivity of the parasympathetic nervous system.
Acknowledgments
This work was supported by the National Science Foundation of China (no. 30770277).
References
- 1.Mersereau WA, Hinchey EJ. Hypothermia-induced gastric hypercontractility in the genesis of the restraint ulcer. Can J Surg. 1981;24:622–625. [PubMed] [Google Scholar]
- 2.Arai I, Muramatsu M, Aihara H. Body temperature dependency of gastric regional blood flow, acid secretion and ulcer formation in restraint and water-immersion stressed rats. Jpn J Pharmacol. 1986;40:501–504. doi: 10.1254/jjp.40.501. [DOI] [PubMed] [Google Scholar]
- 3.Ai HB, Zhang ZD. Studies on the mechanism of gastric mucosal injury induced by water-immersion stress in rats. Acta Physiologica Sinica. 1990;42:496–502. [PubMed] [Google Scholar]
- 4.Ephgrave KS, Cullen JJ, Broadhurst K, Kleiman-Wexler R, Shirazi SS, Schulze-Delrieu K. Gastric contractions, secretions and injury in cold restraint. Neurogastroenterol Motil. 1997;9:187–192. doi: 10.1046/j.1365-2982.1997.d01-42.x. [DOI] [PubMed] [Google Scholar]
- 5.Garrick T, Buack S, Bass P. Gastric motility is a major factor in cold restraint-induced lesion formation in rats. Am J Physiol. 1986;250:G191–G199. doi: 10.1152/ajpgi.1986.250.2.G191. [DOI] [PubMed] [Google Scholar]
- 6.Sagar SM, Sharp SR, Curran T. Expression of c-fos protein in the brain: metabolic mapping at the cellular level. Science. 1988;240:1328–1331. doi: 10.1126/science.3131879. [DOI] [PubMed] [Google Scholar]
- 7.Bonaz B, Tache Y. Induction of Fos immunoreactivity in the rat brain after cold-restraint induced gastric lesions and fecal excretion. Brain Res. 1994;652:56–64. doi: 10.1016/0006-8993(94)90316-6. [DOI] [PubMed] [Google Scholar]
- 8.Wang L, Cardin S, Martínez V, Taché Y. Intracerebroventricular CRF inhibits cold restraint-induced c-fos expression in the dorsal motor nucleus of the vagus and gastric erosions in rats. Brain Res. 1996;736:44–53. doi: 10.1016/0006-8993(96)00726-3. [DOI] [PubMed] [Google Scholar]
- 9.Ma S, Morilak DA. Induction of Fos expression by acute immobilization stress is reduced in locus coeruleus and medial amygdala of Wistar–Kyoto rats compared to Sprague–Dawley rats. Neuroscience. 2004;124:963–972. doi: 10.1016/j.neuroscience.2003.12.028. [DOI] [PubMed] [Google Scholar]
- 10.Chowdhury GMI, Fujioka T, Nakamura S. Induction and adaptation of Fos expression in the rat brain by two types of acute restraint stress. Brain Res Bull. 2000;52:171–182. doi: 10.1016/S0361-9230(00)00231-8. [DOI] [PubMed] [Google Scholar]
- 11.Crane JW, French KR, Buller KM. Patterns of neuronal activation in the rat brain and spinal cord in response to increasing durations of restraint stress. Stress. 2005;8:199–211. doi: 10.1080/10253890500333817. [DOI] [PubMed] [Google Scholar]
- 12.Miyata S, Itoh T, Lin S-H, Ishiyama M, Nakashima T, Kiyohara T. Temporal changes of c-fos expression in oxytocinergic magnocellular neuroendocrine cells of the rat hypothalamus with restraint stress. Brain Res Bull. 1995;37:391–395. doi: 10.1016/0361-9230(95)00017-8. [DOI] [PubMed] [Google Scholar]
- 13.Maruyama M, Matsumoto H, Fujiwara K, Noguchi J, Kitada C, Fujino M, Inoue K. Prolactin-releasing peptide as a novel stress mediator in the central nervous system. Endocrinology. 2001;142:2032–2038. doi: 10.1210/en.142.5.2032. [DOI] [PubMed] [Google Scholar]
- 14.Zhang YY, Cao GH, Zhu WX, Cui XY, Ai HB. Comparative study of c-Fos expression in the rat dorsal vagal complex and nucleus ambiguus induced by different durations of restraint water-immersion stress. Chin J Physiol. 2009;52(3):143–150. doi: 10.4077/cjp.2009.amh045. [DOI] [PubMed] [Google Scholar]
- 15.Guth PH, Aures D, Paulsen G. Topical aspirin plus HCl gastric lesion in the rat. Cytoprotective effect of prostaglandin, cimetidine and probanthine. Gastroenterology. 1979;76:88–93. [PubMed] [Google Scholar]
- 16.Paxinos G, Watson C. The rat brain in stereotaxic coordinates (fifth edition) Burlington: Elsevier Academic Press; 2005. [Google Scholar]
- 17.Holahan MR, White NM. Amygdala c-Fos induction corresponds to unconditioned and conditioned aversive stimuli but not to freezing. Behav Brain Res. 2004;152:109–120. doi: 10.1016/j.bbr.2003.09.031. [DOI] [PubMed] [Google Scholar]
- 18.Ceccatelli S, Villar MJ, Golstein M, Hokfelt T. Expression of c-fos immunoreactivity in transmitter-characterized neurons after stress. Proc Natl Acad Sci USA. 1989;86:9569–9573. doi: 10.1073/pnas.86.23.9569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Pacak K, Palkovits M. Stressor specificity of central neuroendocrine responses: implications for stress-related disorders. Endocr Rev. 2001;22:502–548. doi: 10.1210/er.22.4.502. [DOI] [PubMed] [Google Scholar]
- 20.Yano S, Harada M. Activation of autonomic nervous system during exposure of rats to restraint and water immersion stress. II. Comparison with restraint stress. J Pharmacobiodyn. 1980;3:11–16. doi: 10.1248/bpb1978.3.11. [DOI] [PubMed] [Google Scholar]
- 21.Xie YF, Jiao Q, Guo S, Wang FZ, Cao JM, Zhang ZG. Role of parasympathetic overactivity in water immersion stress-induced gastric mucosal lesion in rat. J Appl Physiol. 2005;99:2416–2422. doi: 10.1152/japplphysiol.00267.2005. [DOI] [PubMed] [Google Scholar]
- 22.Cho CH, Qui BS, Bruce IC. Vagal hyperactivity in stress induced gastric ulceration in rats. J Gastroenterol Hepatol. 1996;11:125–128. doi: 10.1111/j.1440-1746.1996.tb00048.x. [DOI] [PubMed] [Google Scholar]
- 23.Zhang JF, Zheng F, Zhan MC, Sun D, Yan CD. Effect of electrical stimulation of paraventricular nucleus on stress gastric mucosal lesion in rats. Acta Physiol Sin. 1992;44:583–590. [PubMed] [Google Scholar]
- 24.Eugene JZ, Koplovitz I. Gastric secretory response of the anesthetized dog after direct chemical stimulation of the supraoptic region. Exp Neurol. 1977;55:122–132. doi: 10.1016/0014-4886(77)90164-9. [DOI] [PubMed] [Google Scholar]
- 25.Hastings MH, Duffield GE, Ebling FJ, Kidd A, Maywood ES, Schurov I. Non-photic signalling in the suprachiasmatic nucleus. Biol Cell. 1997;89:495–503. doi: 10.1016/S0248-4900(98)80005-1. [DOI] [PubMed] [Google Scholar]
- 26.Brzozowski T, Zwirska-Korczala K, Konturek PC, Konturek SJ, Sliwowski Z, Pawlik M, Kwiecien S, Drozdowicz D, Mazurkiewicz-Janik M, Bielanski W, Pawlik WW. Role of circadian rhythm and endogenous melatonin in pathogenesis of acute gastric bleeding erosions induced by stress. J Physiol Pharmacol. 2007;58(Suppl 6):53–64. [PubMed] [Google Scholar]
- 27.Pitkanen A, Jolkkonen E, Kemppainen S. Anatomic heterogeneity of the rat amygdaloid complex. Folia Morphol (Warsz.) 2000;59:1–23. [PubMed] [Google Scholar]
- 28.Knapska E, Radwanska K, Werka T, Kaczmarek L. Functional internal complexity of amygdale: focus on gene activity mapping after behavioral training and drugs of abuse. Physiol Rev. 2007;87:1113–1173. doi: 10.1152/physrev.00037.2006. [DOI] [PubMed] [Google Scholar]
- 29.Prewitt CM, Herman JP. Anatomical interactions between the central amygdaloid nucleus and the hypothalamic paraventricular nucleus of the rat: a dual tract-tracing analysis. J Chem Neuroanat. 1998;15:173–185. doi: 10.1016/S0891-0618(98)00045-3. [DOI] [PubMed] [Google Scholar]
- 30.Gray TS, Carney ME, Magnuson DJ. Direct projections from the central amygdaloid nucleus to the hypothalamic paraventricular nucleus: possible role in stress-induced adrenocorticotropin release. Neuroendocrinology. 1989;50:433–446. doi: 10.1159/000125260. [DOI] [PubMed] [Google Scholar]
- 31.Liubashina O, Jolkkonen E, Pitkanen A. Projections from the central nucleus of the amygdala to the gastric related area of the dorsal vagal complex: a Phaseolus vulgaris-leucoagglutinin study in rat. Neurosci Lett. 2000;291:85–88. doi: 10.1016/S0304-3940(00)01392-6. [DOI] [PubMed] [Google Scholar]
- 32.Grijalva CV, Tache Y, Gunion MW, Walsh JH, Geiselman PJ. Amygdaloid lesions attenuate neurogenic gastric mucosal erosions but do not alter gastric secretory changes induced by intracistemal bombesin. Brain Res Bull. 1986;16:55–61. doi: 10.1016/0361-9230(86)90012-2. [DOI] [PubMed] [Google Scholar]
- 33.Henke PG. Recent studies of the central nucleus of the amygdala and stress ulcers. Neurosci Biobehav Rev. 1988;12:143–150. doi: 10.1016/S0149-7634(88)80006-X. [DOI] [PubMed] [Google Scholar]
- 34.Henke PG. The centromedial amygdala and gastric pathology in rats. Physiol Behav. 1980;25:107–112. doi: 10.1016/0031-9384(80)90189-4. [DOI] [PubMed] [Google Scholar]
- 35.Innes DK, Tansy MF. Gastric mucosal ulceration associated with electrochemical stimulation of the limbic system. Brain Res Bull. 1980;5(Suppl 1):33–36. doi: 10.1016/0361-9230(80)90300-7. [DOI] [PubMed] [Google Scholar]
- 36.Henke PG. Facilitation and inhibition of gastric pathology after lesions in the amygdala of rats. Physiol Behav. 1980;25:575–579. doi: 10.1016/0031-9384(80)90124-9. [DOI] [PubMed] [Google Scholar]
- 37.Chrousos GP, Gold PW. The concepts of stress and stress system disorders. Overview of physical and behavioral homeostasis. JAMA. 1992;267:1244–1252. doi: 10.1001/jama.267.9.1244. [DOI] [PubMed] [Google Scholar]
- 38.Henke PG. The telencephalic limbic system and experimental gastric pathology, a review. Neurosci Biobehav Rev. 1982;6:381–390. doi: 10.1016/0149-7634(82)90047-1. [DOI] [PubMed] [Google Scholar]


