Abstract
To examine the relationship between specific insulin-like growth factor (IGF)-binding proteins (IGFBPs) and myogenesis during muscle regeneration in vivo, we measured mRNA expression of IGFBPs and myogenic markers in rat plantaris muscle after bupivacaine administration. IGF-I Ea, MGF, IGFBPs and myogenic marker mRNAs were analyzed 12, 24, 48 and 72 h after bupivacaine injection. IGFBP-1, -2, -3 and -4 proteins were immunostained after the treatment. MGF, IGF-I Ea and IGFBP-4 mRNAs started to increase 12 or 24 h after bupivacaine injection and increased further after that. IGFBP-1, -2, -3 and -4 proteins were strongly stained in the immature muscle fiber nuclei and the extracellular matrix after bupivacaine injection. PCNA, MyoD, IGFBP-2 and IGFBP-3 mRNAs increased at 12 or 24 h and did not show further increases after that. Myogenin, p21, IGFBP-1 and IGFBP-5 mRNAs sharply increased after 72 h. These results suggest that specific IGFBPs are individually expressed and differently associated with the expression of myogenic markers in regenerating muscles.
Keywords: Insulin-like growth factor-1, Mechano growth factor, Insulin-like growth factor-binding proteins, Myogenic regulatory factors, Muscle regeneration
Introduction
Insulin-like growth factor-I (IGF-I) is an important growth factor mediating various developmental processes in skeletal muscles and activates cell proliferation, differentiation, cell survival, etc. There are two types of IGF-I isoforms derived from the differential E domain in rodents called IGF-I Ea and IGF-I Eb. IGF-I Eb is also called mechano growth factor (MGF) because of the marked upregulation in exercised and damaged skeletal muscles [1–3]. These IGF-Is are produced by various tissues, including liver, cartilage and skeletal muscle, and act through endocrine and autocrine/paracrine pathways. Most of the circulating IGF-I exists in a large tripartite complex with IGF-binding protein-3 (IGFBP-3) and the acid labile subunit [4]. IGF-I also exists in binary or ternary complexes with another member of the IGFBP family [4]. IGF-I is removed from the complexes, and free IGF-I acts on muscle growth via the IGF-I receptors.
The IGFBP family is composed of six different members: IGFBP-1, IGFBP-2, IGFBP-3, IGFBP-4, IGFBP-5 and IGFBP-6 [5, 6]. Many kinds of tissues express more than one IGFBP [7–9]. Muscle cells are known to produce and secrete several IGFBPs [7, 10]. The large complex of IGF-I in the circulation cannot cross the vascular endothelium unless this complex is broken down and therefore it acts to regulate the endocrine actions of IGF-I. In contrast, the remaining IGF/IGFBP complex easily crosses the vascular endothelium and is thought to be locally bioavailable to the target tissues [4].
The IGFBPs have stimulating and inhibitory effects on IGF-I [11]. IGFBP-1, IGFBP-2, IGFBP-4 and IGFBP-6 inhibit IGF's actions by binding to IGF-I and preventing its binding to their receptors; however, IGFBP-3 and IGFBP-5 stimulate the actions of IGF-I [12]. The same IGFBP can act to potentiate or inhibit IGF's actions depending on various conditions, such as the culture conditions, cell type and IGFBP dose [13–17]. IGFBPs are known to have IGF-independent actions in cell migration, cell growth and apoptosis [4]. Thus, the function of the IGFBP family is complicated and not completely understood.
The IGFBPs in skeletal muscle appear to be expressed individually in response to various stimuli. Jennische and Hall [18] showed that the expression and localization of IGFBP mRNAs differed according to their isoforms in rat regenerating skeletal muscle. Awede et al. [19] also showed that IGFBP-4 mRNA expression was increased in overloading muscle, but not in IGFBP-5 mRNA. In contrast, IGFBP-5 mRNA expression was increased in unloaded muscle but not in IGFBP-4 mRNA. These reports provide for the possibility that each IGFBP is differentially related to muscle regeneration or hypertrophy. However, the expression of IGFBPs in skeletal muscle has not been examined in association with the gene expression of myogenic markers, which are proliferation or differentiation markers. Therefore, the relationship between specific IGFBPs and myogenesis is unclear. Consequently, to examine the relationship between specific IGFBPs and myogenesis during muscle regeneration in vivo, we measured mRNA expression of IGFBPs and myogenic markers in rat plantaris muscle after bupivacaine administration.
Methods
Animal care
Male Wistar rats aged 8 weeks and weighing 180–220 g were used. All animals were housed in cages at room temperature (22 ± 1 °C) and fed ad libitum. Forty animals were given bupivacaine or saline. Five animals were used as the untreated controls. All surgical procedures were performed under anesthesia by intraperitoneal administration of sodium pentobarbital (50 mg kg−1 body weight). All experiments and procedures were conducted according to the Guideline for the Care and Use of Laboratory Animals of the Health Sciences University of Hokkaido.
Bupivacaine administration
The animals were given bupivacaine (n = 20) or saline (n = 20). The bupivacaine (0.5 ml of 0.5 % bupivacaine solution) was administered by intramuscular injections into the plantaris muscles of both hind limbs. The saline administration was performed the same way and served as control. Surgery started with a longitudinal incision through the skin and fascia along the posterior aspect of the tibia, followed by intramuscular administration of bupivacaine or saline using disposable 26.5-gauge needles inserted into the distal part of the plantaris muscle through the belly to the proximal part. The needles were slowly withdrawn while the drugs were injected. At 12, 24, 48 and 72 h after treatment, each rat (n = 5) was killed by cervical dislocation under anesthesia to harvest muscle samples. The plantaris muscles were dissected quickly, freed of any fat and connective tissue, frozen in liquid nitrogen and stored at −80 °C for later mRNA analysis.
Immunohistochemistry
Plantaris muscles were immunostained using rabbit anti-IGFBP-1, anti-IGFBP-2, anti-IGFBP-3 and anti-IGFBP-4 antibodies (PAAH1, PAAl1, PAAJ1 and PAAG1; Cell Sciences, Canton, MA, USA) 72 h after bupivacaine administration. Transverse sections (10 μm) were fixed with 4 % neutral buffered paraformaldehyde. Non-specific protein binding was blocked by PBS containing 10 % normal goat serum for 2 h. Tissue sections were incubated with the primary antibody overnight at room temperature and with a biotinylated anti-rabbit antibody (Vector Laboratories, Burlingame, CA) for 1 h. This was followed by incubation with streptavidin-coupled horseradish peroxidase using an avidin–biotin detection kit (Vectastain ABC kit; Vector Laboratories). The tissue sections were stained with 3,3′-diaminobenzidine (Sigma Fast Tablet; Sigma, St Louis, MO, USA) in the presence of hydrogen peroxide, and after washing in distilled water, they were counterstained with hematoxylin.
Analysis of mRNA expression by using real-time PCR
We used real-time PCR to analyze the expression of mRNAs for IGFBP-1, IGFBP-2, IGFBP-3, IGFBP-4, IGFBP-5, IGFBP-6, IGF-I Ea, mechano growth factor (MGF), MyoD, myogenin, proliferating cell nuclear antigen (PCNA) and p21. Total RNA was extracted using the TRIZOL reagent (Invitrogen, Tokyo, Japan) and the RNA concentrations determined spectrophotometrically at 260 nm. The quality of RNA was checked spectrophotometrically by using the ratio of absorbances at 260/280 nm, and the values of all samples were in the range of 1.8–2.0. After extraction, the RNA samples were treated with TURBO DNA-free™ (Ambion, Austin, TX, USA) for 30 min at 37 °C to remove any genomic DNA. The DNase-treated RNA (0.25 μg) was used to synthesize first-strand cDNA using the PrimeScript™ RT reagent Kit (Takara, Tokyo, Japan). The cDNA products (100 ng μl−1) were analyzed by using real-time PCR using the Power SYBR Green PCR Master Mix (Applied Biosystems, Tokyo, Japan) in an ABI PRISM 7300 (Applied Biosystems). The amplification program included an initial denaturation step at 95 °C for 10 min, 40 cycles of denaturation at 95 °C for 15 s and annealing/extension at 60 °C for 1 min. The level of glyceraldehyde-3-phosphate dehydrogenase (GAPDH) mRNA was estimated for use as an internal control. Melting point analysis did not detect any non-specific amplification in the cDNA samples. The slopes of amplification curves were not different between groups in an mRNA analysis and differences of amplification efficiency were not observed. The sequences of the specific primers used in the Power SYBR Green Master Mix protocol are given in Table 1. Each PCR primer was designed using the ABI primer express software (v 1.5), and the oligonucleotides were purchased from Hokkaido System Science (Sapporo, Japan).
Table 1.
Specific primers used in the real-time RT-PCR
| Gene | Forward primer (5′–3′) | Position | Reverse primer (5′–3′) | Position |
|---|---|---|---|---|
| IGFBP-1 | TGCCGGAGTTCCTAACTGTTGT | 161 | CCAGCGACTACGCGAACCT | 233 |
| IGFBP-2 | GTGATGCCTGGCCCACTTA | 113 | TCGTCTTGATTTCCTGGACAATG | 184 |
| IGFBP-3 | TGCTGGGAGTGTGGAAAGC | 494 | GAGTGGATGGAACTTGGAATCAGT | 563 |
| IGFBP-4 | GCATCCCAACAACAGCTTCA | 607 | ATCTCTCACTTTGGCCATATGCTT | 685 |
| IGFBP-5 | TGTGTGGACAAGTATGGGATGAA | 1274 | AAGGCGTGGCACTGAAAGTC | 1344 |
| IGFBP-6 | TCCCTGCTGGTGTGTGGAT | 593 | GAGAGCTTCCCTGGCCATCT | 660 |
| IGF-I Ea | AGCTGAGATAGTGTTTCCCAAAGG | 116 | TTCCAAACGCGAAATGAATG | 184 |
| MGF | TGACATGCCCAAGACTCAGAAGT | 408 | CCTTCTCCTTTGCAGCTTCCT | 477 |
| MyoD | GCCCGGTCTGCACTCATG | 1193 | GAGTGTCATTTAGCTTCATTTTTGG | 1267 |
| PCNA | TCCGAAGGCTTCGACACATAC | 307 | GGACATGCTGGTGAGGTTCA | 375 |
| myogenin | TGGTACCCAGTGAATGCAACTC | 504 | GGACCAAACTCCAGTGCATTG | 576 |
| p21 | GGCAGACCAGCCTAACAGATTT | 469 | GGCACTTCAGGGCTTTCTCTT | 542 |
| GAPDH | AGACTGTGGATGGCCCCTCT | 622 | GATGACCTTGCCCACAGCCT | 728 |
Statistics
All data were expressed as the mean and standard deviation. A two-way factorial ANOVA was used for comparisons between groups and time points. When the ANOVA results revealed significant interactions, post hoc tests were performed with Tukey’s multiple comparison test. To determine if there were any relationships between the temporal expression of the IGFBPs and myogenic markers during muscle regeneration, Pearson's correlation coefficient between the time course expression of IGFBPs mRNAs and myogenic markers mRNAs after bupivacaine injection was analyzed. Differences between the mean values and correlation coefficient between the mRNAs were regarded as significant when p < 0.05.
Results
IGF-I Ea and MGF mRNA expressions in the plantaris muscles 12, 24, 48 and 72 h after bupivacaine injection are shown in Fig. 1. IGF-I Ea mRNA started to increase 24 h after bupivacaine injection (p < 0.05, Fig. 1a) and increased further after 48 and 72 h. MGF mRNA started to increase 12 h after bupivacaine injection (p < 0.05, Fig. 1b) and increased further after 24, 48 and 72 h.
Fig. 1.
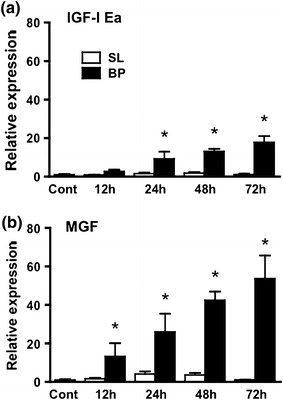
mRNA expression of IGF-I Ea (a) and MGF (b) after bupivacaine injection. BP bupivacaine-injected muscle, SL saline-injected muscle. Data are presented as the relative values compared to those of the untreated control group (cont). Values are the mean ± SD. Significant differences from cont (*p < 0.05)
Figure 2 shows PCNA, MyoD, p21 and myogenin mRNA expression in the plantaris muscles 12, 24, 48 and 72 h after bupivacaine injection. PCNA and MyoD mRNAs started to increase 24 h after bupivacaine injection (p < 0.05, Fig. 2a, b). PCNA mRNA reached the peak level after 24 h and MyoD after 48 h. Myogenin and p21 mRNAs started increasing 48 and 12 h after bupivacaine injection, and they increased further after 72 h (p < 0.05, Fig. 2c, d).
Fig. 2.
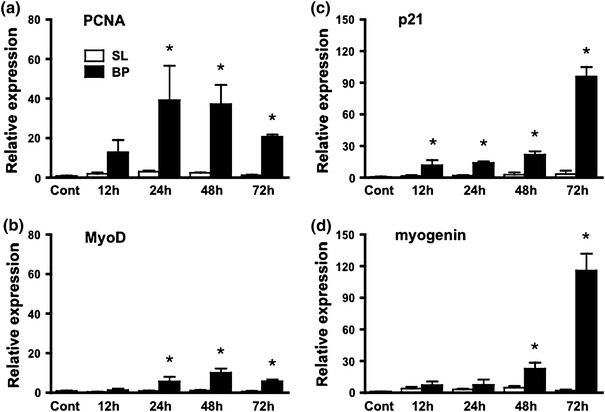
mRNA expression of PCNA (a), MyoD (b), p21 (c) and myogenin (d) after bupivacaine injection. BP bupivacaine-injected muscle, SL saline-injected muscle. Data are presented as the relative values compared to those of the untreated control group (cont). Values are the mean ± SD. Significant differences from cont (*p < 0.05)
Figure 3 shows IGFBP-1, IGFBP-2, IGFBP-3, IGFBP-4, IGFBP-5 and IGFBP-6 mRNA expression in the plantaris muscles 12, 24, 48 and 72 h after bupivacaine injection. IGFBP-2, IGFBP-3 and IGFBP-6 mRNAs were increased 12 h after bupivacaine injection (p < 0.05, Fig. 3b, c, f) and did not increase further. IGFBP-4 mRNA started to increase 24 h after bupivacaine injection (p < 0.05, Fig. 3d) and increased further after 72 h. IGFBP-5 mRNA started increasing 48 h after bupivacaine injection (p < 0.05, Fig. 3e) and increased further after 72 h. IGFBP-1 mRNA was significantly increased 72 h after bupivacaine injection (p < 0.05, Fig. 3a).
Fig. 3.
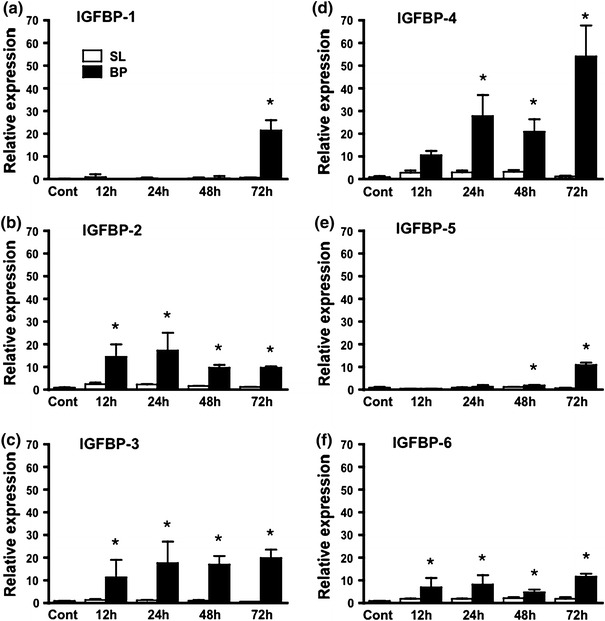
mRNA expression of IGFBP-1 (a), IGFBP-2 (b), IGFBP-3 (c), IGFBP-4 (d), IGFBP-5 (e) and IGFBP-6 (f) after bupivacaine injection. BP bupivacaine-injected muscle, SL saline-injected muscle. Data are presented as the relative values compared to those of the untreated control group (cont). Values are the mean ± SD. Significant differences from cont (*p < 0.05)
There were significant correlations between the time course expression of IGFBP-3 mRNA and those of PCNA (r = 0.667, p < 0.05) and MyoD (r = 0.586, p < 0.05) mRNAs after bupivacaine injection. The IGFBP-2 mRNA was only correlated with the PCNA mRNA (r = 0.499, p < 0.05). The IGFBP-1, IGFBP-4, IGFBP-5 and IGFBP-6 mRNAs were significantly correlated with the p21 (r = 0.955, r = 0.810, r = 0.993 and r = 0.600, p < 0.05) and myogenin (r = 0.955, r = 0.816, r = 0.977 and r = 0.489, p < 0.05) mRNAs.
To identify the localization of IGFBP-1, IGFBP-2, IGFBP-3 and IGFBP-4 proteins, which showed remarkable mRNA expression, were immunostained 72 h after bupivacaine injection (Fig. 4). A large number of regenerating immature muscle fiber nuclei were identified after bupivacaine injection (Fig. 4e). IGFBP-1, IGFBP-2, IGFBP-3 and IGFBP-4 proteins were strongly stained in the immature muscle fiber nuclei and the extracellular matrix after bupivacaine injection (Fig. 4a–d).
Fig. 4.
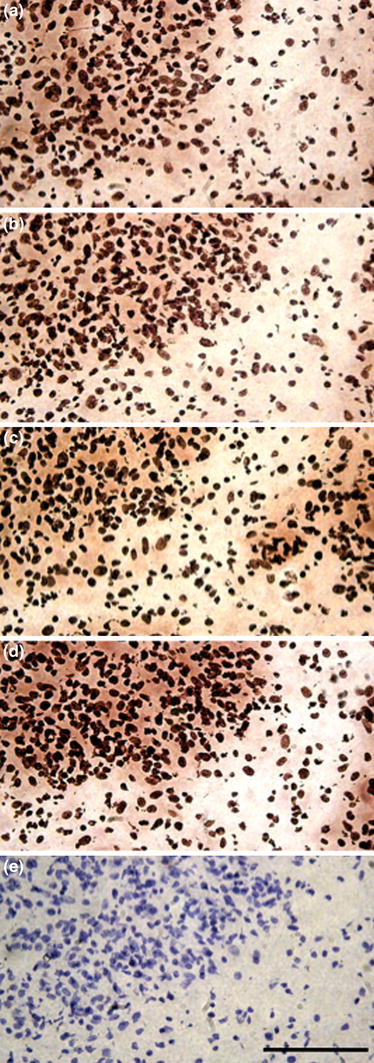
Immunostained sections for IGFBP-1 (a), IGFBP-2 (b), IGFBP-3 (c) and IGFBP-4 (d) proteins 72 h after bupivacaine injection. Tissues were muscle fiber nuclei counterstained with hematoxylin (e). Bar 100 μm
Discussion
Our study showed the expression of specific IGFBPs corresponding with myogenic markers in regenerating muscles in vivo. The mRNA expression pattern of IGFBP-2 and IGFBP-3 was similar to those of PCNA and MyoD mRNAs. The time course expression of IGFBP-3 mRNA after bupivacaine injection was significantly correlated with those of PCNA and MyoD mRNAs. IGFBP-2 mRNA was significantly correlated with PCNA mRNA. In contrast, the expression of IGFBP-1 and IGFBP-5 mRNAs started to increase 72 h after bupivacaine injection and was similar to those of p21 and myogenin mRNAs. The expression of IGFBP-1 and IGFBP-5 mRNAs was significantly correlated with those of p21 and myogenin mRNAs. Activated satellite cells in regenerating muscles express myogenic regulatory factors and several cell cycle markers. When quiescent satellite cells are activated to enter the cell cycle, the activated cells express MyoD, which is a myogenic regulatory factor. In addition, PCNA is expressed in proliferating satellite cells [20]. Expression of myogenin initiated in the activated satellite cells continues through fusion and differentiation [20]. Differentiating satellite cells also express p21, one of the cyclin-dependent kinase inhibitors, which mediates withdrawal from the cell cycle [21, 22]. These observations indicate that IGFBP-2 and IGFBP-3 are associated with the proliferating process in regenerating muscles and that IGFBP-1 and IGFBP-5 are related to the differentiation process but not to proliferation. James et al. [10] showed that IGFBP-5 is expressed during myoblast differentiation in a culture system. Ren et al. [23] showed that knockdown of IGFBP-5 inhibits myogenic differentiation in vitro. These reports are in line with the present observations. In vitro studies have demonstrated that IGF-I upregulated cell proliferation and caused a satellite cell differentiation process [24, 25]. The mechanism by which IGF-I induces both proliferation and differentiation in skeletal muscle is obscure. The differential expression of specific IGFBP isoforms may be associated with the switching from proliferation to differentiation in regenerating muscles.
Although IGFBPs have either inhibited or stimulated IGF actions, all members of the IGFBPs in this study were upregulated during the regenerating process after muscle damage. Awede et al. [19] showed that IGFBP-4 mRNA expression increased in overloading muscle but not IGFBP-5 mRNA. In contrast, Awede et al. [26] showed that IGFBP-4 and IGFBP-5 mRNA was increased by clenbutenol-induced muscle hypertrophy. Jennische and Hall [18] also showed that IGFBP-3, IGFBP-4, IGFBP-5 and IGFBP-6 mRNAs were upregulated in rat regenerating skeletal muscle.
IGFBP-4's inhibition of IGF's actions by binding to IGF-I and preventing the binding of IGF-I to their receptors is well known [4, 11]. In the present study, IGFBP-4 mRNA was potentiated in regenerating muscles, and IGFBP-4 proteins were strongly immunostained in immature muscle fiber nuclei and the extracellular matrix of regenerating muscles. As the same IGFBP could act to potentiate or inhibit IGF actions depending on various conditions such as culture conditions, cell type and IGFBP dose [13–16], IGFBP-4 in vivo may have different functions on IGF actions. The increase of IGFBP-4 protein in the extracellular matrix may contribute to the concentration of local IGF-I levels around the target tissues. As the IGFBPs' binding to IGF-I is known to increase the longevity of IGF-I [27], the increased expression of IGFBP-4 may be associated with the continuing actions of IGF-I for the target tissues. The potentiation of the actions of IGF-I by IGFBP-5 in smooth muscle cells has been shown to involve the binding of IGFBP-5 to the extracellular matrix [15]. Locally produced IGFBPs would act as autocrine-paracrine regulators of IGF actions in regenerating muscles.
The timing of IGFBP-2, IGFBP-3 and IGFBP-6 mRNA expression was the same as that of MGF mRNA, indicating that they were associated with the action of MGF. MGF is a member of IGF-I specifically expressed in exercised and damaged skeletal muscles [1–3]. In rodents, Hill and Goldspink [1] showed that the timing of IGF-I mRNA expression in response to mechanical and pharmacological damages differs among IGF-I isoforms and that the expression of MGF mRNA precedes that of IGF-I Ea. In humans, McKay et al. [28] demonstrated that MGF mRNA was expressed earlier than those of IGF-I Ea and IGF-I Eb after exercise-induced muscle damage. McKay et al. [28] suggested that the temporal expression of MGF was related to the activation and proliferative phase of myogenic processes, as MGF expression was strongly correlated with the increases of MyoD and myf5. In cultures of C2/C12 skeletal muscle cells treated with IGF-I Ea and MGF, Yang and Goldspink [29] demonstrated that IGF-I Ea-treated cells initiated the fusion of myoblasts to form myotubes and that MGF-treated cells showed evidence of proliferation but remained in the mononucleated state. Thus, IGFBP-2, IGFBP-3 and IFGBP-6 may be associated with the proliferating actions of MGF in regenerating muscles.
In the present study, the mRNA expression patterns of the IGFBP family, except IGFBP-4 mRNA, were different from those of IGF-I. This suggests that the expression of IGFBPs is not necessarily controlled by IGF-I. IGFBPs are known to be under the control of both systemic hormones, including growth hormone, parathyroid hormone, glucocorticoid, etc., and local regulators, including TGF-ß, interleukins, etc. [4]. In addition, IGFBPs have IGF-I-independent actions in cell migration, cell growth and apoptosis [4]. IGF-I-independent expression of IGFBPs in this study may reflect that IGFBPs serve as a growth factor independently of IGF-I in regenerating muscles.
In the present study, the expression of six IGFBP isoforms potentiated during regenerating process after muscle damage. The expression of IGFBP-2 and IGFBP-3 mRNAs is similar to those of the proliferation markers. The expression of IGFBP-1 and IGFBP-5 mRNAs is similar to those of the differentiation markers. IGFBP-4 mRNA is continuously expressed during the proliferation and differentiation processes in regenerating muscles. These observations indicate that each isoform of the IGFBP family is individually expressed and acts differentially on the regenerating processes after muscle damage. The experimental approach in the present study mainly limited the data of the mRNA analysis. The expression of mRNAs may not always reflect that of the proteins. Further experiments are needed to reach an absolute understanding.
References
- 1.Hill M, Goldspink G. Expression and splicing of the insulin-like growth factor gene in rodent muscle is associated with muscle satellite (stem) cell activation following local tissue damage. J Physiol. 2003;549:409418. doi: 10.1113/jphysiol.2002.035832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Hameed M, Orrell RW, Cobbold M, Goldspink G, Harridge DR. Expression of IGF-I splicing variants in young and old human skeletal muscle after high resistance exercise. J Physiol. 2003;547:247–254. doi: 10.1113/jphysiol.2002.032136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Owino V, Yang SY, Goldspink G. Age-related loss of skeletal muscle function and the inability to express the autocrine form of insulin-like growth factor-1 (MGF) in response to mechanical overload. FEBS Lett. 2001;505:259–263. doi: 10.1016/S0014-5793(01)02825-3. [DOI] [PubMed] [Google Scholar]
- 4.Mohan S, Baylink DJ. IGF-binding proteins are multifunctional and act via IGF-dependent and -independent mechanisms. J Endocrinol. 2002;175:19–31. doi: 10.1677/joe.0.1750019. [DOI] [PubMed] [Google Scholar]
- 5.Sara VR, Hall K. Insulin-like growth factors and their binding proteins. Physiol Rev. 1990;70:591–614. doi: 10.1152/physrev.1990.70.3.591. [DOI] [PubMed] [Google Scholar]
- 6.Jones JJ, Clemmons DR. Insulin-like growth factors and their binding proteins: biological actions. Endocr Rev. 1995;16:3–34. doi: 10.1210/edrv-16-1-3. [DOI] [PubMed] [Google Scholar]
- 7.Funk B, Kessler U, Eisenmenger W, Hansmann A, Kolb HJ, Kiess W. The expression of insulin-like growth factor binding proteins is tissue specific during human fetal life and early infancy. Acta Endocrinol (Copenh) 1992;127:107–114. doi: 10.1530/acta.0.1270107. [DOI] [PubMed] [Google Scholar]
- 8.Shimasaki S, Ling N. Identification and molecular characterization of insulin-like growth factor binding proteins (IGFBP-1, -2, -3, -4, -5, -6) Prog Growth Factor Res. 1991;3:243–266. doi: 10.1016/0955-2235(91)90003-M. [DOI] [PubMed] [Google Scholar]
- 9.Schuller AGP, Zwarthff EC, Drop SLS. Gene expression of the six insulin-like growth factor binding proteins in the mouse conceptus during mid- and late gestation. Endocrinology. 1993;132:2544–2550. doi: 10.1210/en.132.6.2544. [DOI] [PubMed] [Google Scholar]
- 10.James PL, Jones SB, Busby WH, Jr, Clemons DR, Rotwein P. A highly conserved insulin-like growth factor-binding protein (IGFBP-5) is expressed during myoblast differentiation. J Biol Chem. 1993;268:22305–22312. [PubMed] [Google Scholar]
- 11.Firth SM, Baxter RC. Cellular actions of the insulin-like growth factor binding proteins. Endocr Rev. 2002;23:624–654. doi: 10.1210/er.2001-0033. [DOI] [PubMed] [Google Scholar]
- 12.Govoni KE, Baylink DJ, Mohan S. The multi-functional role of insulin-like growth factor binding proteins in bone. Pediatr Nephrol. 2005;20:261–268. doi: 10.1007/s00467-004-1658-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kelley KM, Oh Y, Gargosky SE, Gucev Z, Matsumoto T, Hwa V, Ng L, Simpson DM, Rosenfeld RG. Insulin-like growth factor-binding proteins (IGFBPs) and their regulatory dynamics. Int J Biochem Cell Biol. 1996;28:619–637. doi: 10.1016/1357-2725(96)00005-2. [DOI] [PubMed] [Google Scholar]
- 14.Rajaram S, Baylink DJ, Mohan S. Insulin-like growth factor-binding proteins in serum and other biological fluids: regulation and functions. Endocr Rev. 1997;18:801–831. doi: 10.1210/er.18.6.801. [DOI] [PubMed] [Google Scholar]
- 15.Clemmons DR. Role of insulin-like growth factor binding proteins in controlling IGF actions. Mol Cell Endocrinol. 1998;140:19–24. doi: 10.1016/S0303-7207(98)00024-0. [DOI] [PubMed] [Google Scholar]
- 16.Baxter RC. Insulin-like growth factor (IGF)-binding proteins: interactions with IGFs and intrinsic bioactivities. Am J Physiol. 2000;278:E967–E976. doi: 10.1152/ajpendo.2000.278.6.E967. [DOI] [PubMed] [Google Scholar]
- 17.Collett-Solberg PF, Cohen P. Genetics, chemistry, and function of the IGF/IGFBP system. Endocrine. 2000;12:121–136. doi: 10.1385/ENDO:12:2:121. [DOI] [PubMed] [Google Scholar]
- 18.Jennische E, Hall CM. Expression and localization of IGF-binding protein mRNAs in regenerating rat skeletal muscle. Acta Pathol Microbiol Immunol Scand. 2000;108:747–755. doi: 10.1034/j.1600-0463.2000.d01-24.x. [DOI] [PubMed] [Google Scholar]
- 19.Awede B, Thissen J-P, Gailly P, Lebacq J. Regulation of IGF-I, IGFBP-4 and IGFBP-5 gene expression by loading in mouse skeletal muscle. FEBS Lett. 1999;461:263–267. doi: 10.1016/S0014-5793(99)01469-6. [DOI] [PubMed] [Google Scholar]
- 20.Sabourin LA, Rudnicki MA. The molecular regulation of myogenesis. Clin Genet. 2000;57:16–25. doi: 10.1034/j.1399-0004.2000.570103.x. [DOI] [PubMed] [Google Scholar]
- 21.Wang J, Walsh K. Resistance to apoptosis conferred by cdk inhibitors during myocyte differentiation. Science. 1996;273:359–361. doi: 10.1126/science.273.5273.359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Zhang P, Wong C, Liu D, Finegold M, Harper JW, Elledge SJ. p21CIP1 and p57KIP2 control muscle differentiation at the myogenin step. Genes Dev. 1999;13:213–224. doi: 10.1101/gad.13.2.213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ren H, Yin P, Duan C. IGFBP-5 regulates muscle cell differentiation by binding to IGF-II and switching on the IGF-II auto-regulation loop. J Cell Biol. 2008;182:979–991. doi: 10.1083/jcb.200712110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Rosenthal SM, Cheng Z-Q. Opposing early and late effects of insulin-like growth factor on differentiation and the cell cycle regulatory retinoblastoma protein in skeletal myoblasts. Proc Natl Acad Sci USA. 1995;92:10307–10311. doi: 10.1073/pnas.92.22.10307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Engert JC, Berglund EB, Rosenthal N. Proliferation precedes differentiation in IGF-I-stimulated myogenesis. J Cell Biol. 1996;135:431–440. doi: 10.1083/jcb.135.2.431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Awede BL, Thissen J-P, Lebacq J. Role of IGF-I and IGFBPs in the changes of mass and phenotype induced in rat soleus muscle by clenbuterol. Am J Physiol Endocrinol Metab. 2002;282:E31–E37. doi: 10.1152/ajpendo.2002.282.1.E31. [DOI] [PubMed] [Google Scholar]
- 27.Zapf J. Physiological role of the insulin-like growth factor binding proteins. Eur J Endocrinol. 1995;132:645–654. doi: 10.1530/eje.0.1320645. [DOI] [PubMed] [Google Scholar]
- 28.McKay BR, O’Reilly CE, Phillips SM, Tarnopolsky MA, Parise G. Co-expression of IGF-1 family members with myogenic regulatory factors following acute damaging muscle-lengthening contractions in humans. J Physiol. 2008;586:5549–5560. doi: 10.1113/jphysiol.2008.160176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Yang SY, Goldspink G. Different roles of the IGF-I Ec peptide (MGF) and mature IGF-I in myoblast proliferation and differentiation. FEBS Lett. 2002;522:156–160. doi: 10.1016/S0014-5793(02)02918-6. [DOI] [PubMed] [Google Scholar]


