Fig. 1.
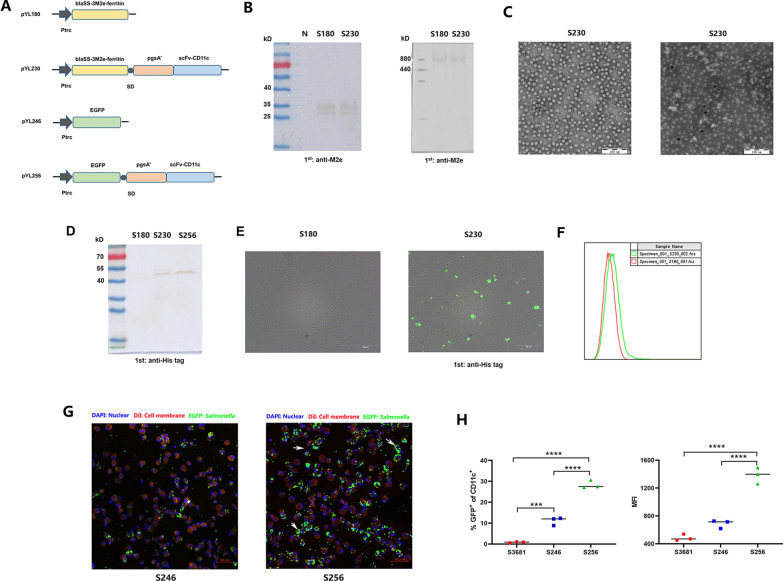
Plasmid construction, protein synthesis and characteristics of the single chain variable fragment of the anti-CD11c (scFv-CD11c) antibody. Plasmid pYL180, encoding three copies of M2e (H1N1) (3M2e), and pYL246, encoding an EGFP fluorescent protein, were used as parental plasmids. Then, a Shine‒Dalgarno (SD) sequence together with the pgsA’ anchoring sequence and scFv-CD11c sequence was inserted into the SacII site, yielding pYL230 and pYL256, respectively (A). Synthesis of 3M2e in Salmonella harboring plasmids pYL180 and pYL230, named S180 and S230, respectively, was confirmed by reducing (left) and non-reducing (right) western blot using an M2e-specific antibody as the primary antibody (B). Purified nanoparticles from S180 and S230 were subjected to a transmission electron microscopy (TEM) assay (left) and immune electron microscopy (right) using an M2e-specific antibody as the primary antibody (C).The production of surface-displayed scFv-CD11c in S230 and Salmonella containing pYL256 (S256) was also determined using a His-tag antibody by western blot (D). Surface-displayed scFv-CD11c on S230 was evaluated by immunofluorescence analysis using His-tag primary antibody and FITC-labeled secondary antibody, S180 was also included as a mock control (E). The presence of scFv-CD11c on the surface of S230 was also confirmed by flow cytometry (F). Mouse bone marrow-derived cells (BMDCs) were incubated with S246 and S256 for 2 h, and then the cells were detected by microscopy. Blue, DAPI-labeled nuclei; red, DiI-labeled cell membrane; green, Salmonella producing EGFP (G). The cellular uptake of Salmonella S246 and S256 was also determined by flow cytometry. The percentages of CD11c+EGFP+ BMDCs as well as the mean fluorescence intensity were calculated (H) (n = 3, ***P < 0.001, ****P < 0.0001)