Abstract
Background
Lymphocytic choriomeningitis virus (LCMV) is a human pathogen naturally present in wild rodents. In addition, LCMV is routinely used in immunology research as a model of viral infection in mice. The Armstrong common laboratory strain and the Clone-13 variant induce acute and chronic infections in mice, respectively. The frequent use of this virus in laboratory settings is associated with a risk of human infection for laboratory personnel. In contrast to LCMV Clone-13, few human laboratory infections with LCMV Armstrong have been reported, leading to a poor understanding of symptoms related to infection with this specific LCMV strain.
Case presentation
A researcher accidentally infected herself percutaneously with LCMV Armstrong. Symptoms including headaches, dizziness, eye pain and nausea appeared seven days post-exposure and lasted ten days. LCMV-IgM antibodies were detected at 28 days post-infection and IgG seroconversion was observed later. Complete recovery was confirmed three months post exposure.
Conclusions
Research involving live viruses comes with the risk of infection for research personnel. This case is the first reported accidental human infection with LCMV Armstrong. The symptoms differed from reported infections with LCMV Clone-13, by the absence of fever and vomiting, and presence of leg numbness. This report will therefore help clinicians and public health authorities to recognize the symptoms associated with LCMV Armstrong infections and to offer appropriate counselling to individuals who accidentally expose themselves.
Keywords: LCMV, Armstrong, Laboratory accidental exposure
Background
Lymphocytic choriomeningitis virus (LCMV) is an enveloped RNA virus that belongs to the Arenaviridae family and its natural host and reservoir is the house mouse (Mus musculus) [1]. It was discovered in 1933 by Charles Armstrong, in St-Louis, when he studied epidemic encephalitis [2–4]. LCMV estimated seroprevalence in the general population ranges between 2 and 20%, depending on the geographic location [2].
Most human LCMV infections occur through exposure to infected rodent excretions via the respiratory tract of the host, where LCMV replicates, moves to the bloodstream, and invades multiple organs [5]. The virus may eventually reach the brain, more specifically the choroid plexus, the ventricular ependymal linings and leptomeninges, where it can replicate to high titers [6]. Typically, LCMV infections in humans will cause an early wave of acute symptoms, followed by a more severe second wave, one-month post-infection [5, 7, 8].
In research laboratories, the Armstrong LCMV strain is routinely used as prototypical models of viral infections in mice. While the Armstrong strain generates an acute viral infection in mice, the Clone-13 variant, a derivative of the Armstrong strain, causes a more chronic infection [9]. In accidental infections of immunocompetent laboratory personnel with LCMV, about one third of individuals are asymptomatic, while others develop flu-like symptoms [2]. In most severe cases, meningitis and encephalitis have been described [7, 10, 11], with a mortality rate of less than 1% [6]. The long-term effects of LCMV Clone-13 infection are still unclear. To our knowledge, no cases of accidental human LCMV Armstrong infection have been reported, limiting our understanding of symptoms related to infection via the percutaneous route. Here, we report the case of an accidental LCMV Armstrong infection in an immunocompetent laboratory worker.
Case presentation
In May 2023, a 25-year-old female researcher living in Montreal presented to the emergency department following an accident, as per her workplace requirements for all work-related accidents involving body fluids. While she was injecting LCMV Armstrong into the peritoneal cavity of mice to study antiviral T cell response, she accidentally injected her finger. This percutaneous accident with a needle containing a solution of LCMV Armstrong was her first exposure to the virus. Other than a cerebral venous sinus thrombosis, which occurred in 2015, her past medical history was unremarkable, with no medical condition causing potential immunosuppression.
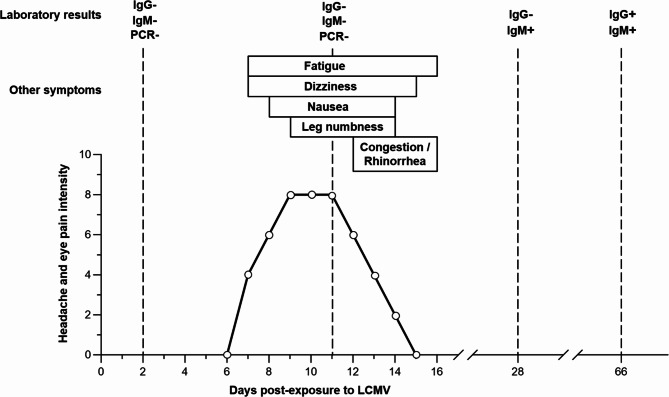
At this first visit, two days (D2) post-exposure, physical examination was unremarkable. The LCMV RNA PCR [12] and serology (IgM and IgG by ELISA [13]), performed at the National Microbiology Laboratory (NML; Winnipeg, Canada), were negative. As detailed in Fig. 1, symptoms started on D7. On D10, she went to the ophthalmology department complaining of very intense eye pain, headaches, dizziness, and nausea. No lesions were observed on magnetic resonance imaging (MRI) of the brain, other than the previously mentioned cerebral venous sinus thrombosis sequelae. Due to the mildness of symptoms, a lumbar puncture was not performed. On D11, following the recommendation of the ophthalmologist, the worker returned to the hospital for a follow-up. She had numbness in her left leg, bilateral eye pain with movement, headache, fatigue, dizziness, and nausea, but no vomiting (Fig. 1). Physical examination was unremarkable. A second blood sample was drawn for both LCMV RNA PCR and serology; the results were again negative (Fig. 1). Additional blood tests were performed at D28 and D66. IgM antibodies to LCMV were detected at D28, with a titer of 1:1600. At D66, LCMV-specific IgM antibodies were still present and IgG seroconversion was observed, with a titer of 1:400 for both antibodies. Three months after the incident, full recovery was confirmed, with no residual symptom.
Fig. 1.
Timeline of symptoms and blood test results following LCMV exposure. Symptoms such as headache and eye pain appeared seven days post-infection, with a severity that was highest between day 9 and day 11 post-infection. Other symptoms included fatigue, dizziness, nausea, leg numbness, congestion, and runny nose. All symptoms lasted for less than ten days. Presence of LCMV-specific IgM was first detected at day 28 post-infection and IgG antibodies were detected at day 66 post-infection. PCR testing was only performed at early time points and was negative
Discussion and conclusions
In the absence of an alternative explanation for the neurological symptoms, this case supports that LCMV infection via percutaneous injection can cause neurological symptoms in an LCMV naïve individual. It also demonstrates that seroconversion can take more than one month and can occur once all the symptoms have dissipated. We recommend testing for seroconversion at days 30 and 60 post-exposure to confirm the infection status of the individual.
Of interest, the Armstrong strain of LCMV, as well as the Clone-13 variant, is considered as risk group 2 pathogens by the Public Health Agency of Canada (https://health.canada.ca/en/epathogen). In this specific case, the infection occurred in a containment level 2 animal facility, where the standard operating procedures include the use of a second pair of gloves and protective sleeves, as well as clear written procedures regarding the handling of mice on the biosafety cabinets. As the symptoms associated with LCMV infection in humans are relatively mild in immunocompetent individuals (Fig. 1) [2, 14, 15], we argue that the containment level 2 is adequate for performing experiments with the Armstrong strain of LCMV. Moreover, as the risk of self-injection is still present in a containment level 3 facility, the classification of LCMV Armstrong as a level 3 pathogen would not significantly reduce the risk of infection for laboratory workers.
Of note, this case differs from natural infections with LCMV, both in terms of dose and route of administration. The solution prepared for injecting mice contained 2 × 105 plaque-forming units (PFU) per milliliter of LCMV Armstrong and exposure was through a percutaneous wound. Although it is difficult to determine precisely, typical needle-stick injuries can inject between 0.3 µL and 6 µL of volume depending on the gauge and depth of needle exposure [16]. Extrapolating this information to this case leads to an infectious dose of LCMV between 60 and 1200 PFU. The higher viral titer in the inoculum, as well as the percutaneous route of exposure, likely explains the severity of the symptoms. Of interest, in contrast to natural exposure via the respiratory tract, the acute response in this patient was not followed by a second wave of aggravated symptoms.
LCMV is an endemic virus throughout all temperate regions of the world. It is estimated that ~ 10% of wild mice are infected with LCMV, although this number may be higher in some settings [17, 18]. One study reported that approximately 5% of the human population carries antibodies against LCMV [2], but the number of people that have been exposed is likely significantly higher. Studies from the 1960s showed that LCMV was one of the most common causes of aseptic meningitis [19], although the proportion of LCMV meningitis reports has recently declined. This latter point is emphasized by the fact that the NML, which is responsible for all LCMV diagnostics in Canada, only runs ~ 50–60 tests for LCMV per year and only 5 positive cases were found over the last 16 years.
In previous case reports and epidemiological studies of laboratory personnel, LCMV infections were related to the Clone-13 variant or to an unknown strain [14, 15, 20–23]. Common symptoms included fever, severe headaches, flu-like symptoms, vomiting, and, in one case, meningitis [14, 15, 20, 21]. More recently, two cases of LCMV Clone-13 infection through a percutaneous route were reported [7, 8]. The two patients developed neck pain, photophobia, nausea, vomiting, flu-like symptoms, pain in the limbs, and fever [7, 8]. This highlights obvious clinical similarities between LCMV Armstrong and LCMV Clone-13 percutaneous infections, with a possible difference in symptom intensity that could be due to differences in viral doses administered or strain type.
The case presented here is unique given the percutaneous route of exposure and the serological evidence of a primary infection. The previous history of cerebral venous sinus thrombosis makes the differential diagnosis of the patient’s original presentation a little broader, although the absence of neurological symptoms in the years preceding LCMV exposure suggests that a contribution from previous brain lesions is unlikely. Symptoms in a seroconverted individual will likely differ from the symptoms reported here, both in severity and duration. This hypothesis should be confirmed in future reports. This case report offers a framework to investigate and follow patients exposed to LCMV Armstrong, filling a gap in our understanding of LCMV as an endemic pathogen and as a laboratory hazard.
Acknowledgements
We thank Cédric Carli for providing information and communicating with the Public Health Agency of Canada.
Abbreviations
- LCMV
Lymphocytic choriomeningitis virus
- MRI
Magnetic resonance imaging
- NML
National Microbiology Laboratory
- PFU
Plaque forming units
Author contributions
LC: investigation, visualization, writing-original draft. JSD: conceptualization, writing-review & editing. JES: validation, writing-review & editing. YD: investigation, writing-review & editing. FLV: investigation, writing-review & editing. ACL: visualization, writing-review & editing, supervision. SL: conceptualization, writing-review & editing, supervision.
Funding
The authors declare that no funding was received for this work. JSD holds the title of Chercheur clinicien chevronné (senior clinical researcher) from Fonds de recherche du Québec – Santé. SL is Research Scholar Emeritus awardee from Fonds de recherche du Québec – Santé.
Data availability
Data sharing is not applicable to this article as no datasets were generated or analyzed during the current study.
Declarations
Competing interests
The authors declare no competing interests.
Ethics approval and consent to participate
This incident has been reported to the Public Health Agency of Canada, as required by local legislation. The ethics committee of the Hôpital Maisonneuve-Rosemont, overseen by the Canadian Council for Animal Protection, approved the experimental procedures in mice (protocol # 2021–2358).
Footnotes
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Contributor Information
Annie-Claude Labbé, Email: ac.labbe@umontreal.ca.
Sylvie Lesage, Email: sylvie.lesage@umontreal.ca.
References
- 1.Traub E. A Filterable Virus recovered from White Mice. Science. 1935;81(2099):298–9. doi: 10.1126/science.81.2099.298. [DOI] [PubMed] [Google Scholar]
- 2.Vilibic-Cavlek T, Savic V, Ferenc T, Mrzljak A, Barbic L, Bogdanic M, et al. Lymphocytic choriomeningitis-emerging trends of a neglected virus: a narrative review. Trop Med Infect Dis. 2021;6(2):88. [DOI] [PMC free article] [PubMed]
- 3.MUCKENFUSS RS, ARMSTRONG C, WEBSTER LT. ETIOLOGY OF THE 1933 EPIDEMIC OF ENCEPHALITIS. JAMA. 1934;103(10):731–3. doi: 10.1001/jama.1934.02750360007004. [DOI] [Google Scholar]
- 4.Armstrong C, Lillie RD. Experimental lymphocytic choriomeningitis of monkeys and mice produced by a Virus encountered in studies of the 1933 St. Louis Encephalitis Epidemic. Public Health Rep (1896-1970) 1934;49(35):1019–27. doi: 10.2307/4581290. [DOI] [Google Scholar]
- 5.Emonet S, Retornaz K, Gonzalez JP, de Lamballerie X, Charrel RN. Mouse-to-human transmission of variant lymphocytic choriomeningitis virus. Emerg Infect Dis. 2007;13(3):472–5. doi: 10.3201/eid1303.061141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bonthius DJ. The arenaviruses. In: Reiss CS, editor. Neurotropic viral Infections: volume 1: neurotropic RNA viruses. Cham: Springer International Publishing; 2016. pp. 149–74. [Google Scholar]
- 7.Aebischer O, Meylan P, Kunz S, Lazor-Blanchet C. Lymphocytic choriomeningitis virus Infection induced by percutaneous exposure. Occup Med (Lond) 2016;66(2):171–3. doi: 10.1093/occmed/kqv156. [DOI] [PubMed] [Google Scholar]
- 8.Drager S, Marx AF, Pigny F, Cherpillod P, Eisermann P, Sendi P, et al. Lymphocytic choriomeningitis virus Meningitis after needlestick injury: a case report. Antimicrob Resist Infect Control. 2019;8:77. doi: 10.1186/s13756-019-0524-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Zhou X, Ramachandran S, Mann M, Popkin DL. Role of lymphocytic choriomeningitis virus (LCMV) in understanding viral immunology: past, present and future. Viruses. 2012;4(11):2650–69. doi: 10.3390/v4112650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Pedrosa PB, Cardoso TA. Viral Infections in workers in hospital and research laboratory settings: a comparative review of Infection modes and respective biosafety aspects. Int J Infect Dis. 2011;15(6):e366–76. doi: 10.1016/j.ijid.2011.03.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Bonthius DJ. Lymphocytic choriomeningitis virus: an underrecognized cause of neurologic Disease in the fetus, child, and adult. Semin Pediatr Neurol. 2012;19(3):89–95. doi: 10.1016/j.spen.2012.02.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Bowen MD, Peters CJ, Nichol ST. Phylogenetic analysis of the Arenaviridae: patterns of virus evolution and evidence for cospeciation between arenaviruses and their rodent hosts. Mol Phylogenet Evol. 1997;8(3):301–16. doi: 10.1006/mpev.1997.0436. [DOI] [PubMed] [Google Scholar]
- 13.Childs JE, Glass GE, Ksiazek TG, Rossi CA, Oro JG, Leduc JW. Human-rodent contact and Infection with lymphocytic choriomeningitis and Seoul viruses in an inner-city population. Am J Trop Med Hyg. 1991;44(2):117–21. doi: 10.4269/ajtmh.1991.44.117. [DOI] [PubMed] [Google Scholar]
- 14.Knust B, Stroher U, Edison L, Albarino CG, Lovejoy J, Armeanu E, et al. Lymphocytic choriomeningitis virus in employees and mice at multipremises feeder-rodent operation, United States, 2012. Emerg Infect Dis. 2014;20(2):240–7. doi: 10.3201/eid2002.130860. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Baum SG, Lewis AM, Jr, Rowe WP, Huebner RJ. Epidemic nonmeningitic lymphocytic-choriomeningitis-virus Infection. An outbreak in a population of laboratory personnel. N Engl J Med. 1966;274(17):934–6. doi: 10.1056/NEJM196604282741704. [DOI] [PubMed] [Google Scholar]
- 16.Mast ST, Woolwine JD, Gerberding JL. Efficacy of gloves in reducing blood volumes transferred during simulated needlestick injury. J Infect Dis. 1993;168(6):1589–92. doi: 10.1093/infdis/168.6.1589. [DOI] [PubMed] [Google Scholar]
- 17.Childs JE, Glass GE, Korch GW, Ksiazek TG, Leduc JW. Lymphocytic choriomeningitis virus Infection and house mouse (Mus musculus) distribution in urban Baltimore. Am J Trop Med Hyg. 1992;47(1):27–34. doi: 10.4269/ajtmh.1992.47.27. [DOI] [PubMed] [Google Scholar]
- 18.Lledo L, Gegundez MI, Saz JV, Bahamontes N, Beltran M. Lymphocytic choriomeningitis virus Infection in a province of Spain: analysis of sera from the general population and wild rodents. J Med Virol. 2003;70(2):273–5. doi: 10.1002/jmv.10389. [DOI] [PubMed] [Google Scholar]
- 19.Meyer HM, Jr, Johnson RT, Crawford IP, Dascomb HE, Rogers NG. Central nervous system syndromes of vital etiology. A study of 713 cases. Am J Med. 1960;29:334–47. doi: 10.1016/0002-9343(60)90029-2. [DOI] [PubMed] [Google Scholar]
- 20.Armstrong D, Fortner JG, Rowe WP, Parker JC. Meningitis due to lymphocytic choriomeningitis virus endemic in a hamster colony. JAMA. 1969;209(2):265–7. doi: 10.1001/jama.1969.03160150051019. [DOI] [PubMed] [Google Scholar]
- 21.Dykewicz CA, Dato VM, Fisher-Hoch SP, Howarth MV, Perez-Oronoz GI, Ostroff SM, et al. Lymphocytic choriomeningitis outbreak associated with nude mice in a research institute. JAMA. 1992;267(10):1349–53. doi: 10.1001/jama.1992.03480100055030. [DOI] [PubMed] [Google Scholar]
- 22.Gregg MB. Recent outbreaks of lymphocytic choriomeningitis in the United States of America. Bull World Health Organ. 1975;52(4–6):549–53. [PMC free article] [PubMed] [Google Scholar]
- 23.Hotchin J. The contamination of laboratory animals with lymphocytic choriomeningitis virus. Am J Pathol. 1971;64(3):747–69. [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Data sharing is not applicable to this article as no datasets were generated or analyzed during the current study.