Abstract
Salivary secretion displays day–night variations that are controlled by the circadian clock. The central clock in the suprachiasmatic nucleus (SCN) regulates daily physiological rhythms by prompting peripheral oscillators to adjust to changing environments. Aquaporin 5 (Aqp5) is known to play a key role in salivary secretion, but the association between Aqp5 and the circadian rhythm is poorly understood. The aim of our study was to evaluate whether Aqp5 expression in submandibular glands (SMGs) is driven by the central clock in the SCN or by autonomous oscillations. We observed circadian oscillations in the activity of period circadian protein homolog 2 and luciferase fusion protein (PER2::LUC) in cultured SMGs with periodicity depending on core clock genes. A daily rhythm was detected in the expression profiles of Aqp5 in SMGs in vivo. In cultured SMGs ex vivo, clock genes showed distinct circadian rhythms, whereas Aqp5 expression did not. These data indicate that daily Aqp5 expression in the mouse SMG is driven by the central clock in the SCN.
Keywords: Circadian rhythm, Salivary gland, Suprachiasmatic nucleus, Aquaporin 5, Period 2
Introduction
Saliva is essential for the maintenance of oral homeostasis [1, 2] and contains various enzymes and growth factors [3, 4]. In humans, saliva is secreted from three major glands, namely, the parotid glands, sublingual glands, and submandibular glands (SMGs), as well as other minor glands [5]. Saliva secretion is regulated by the autonomic nervous system through different mechanisms. For example, sympathetic stimulation greatly increases salivary protein concentration while lowering the flow rate, while parasympathetic stimulation triggers an abundant flow of saliva with a lower protein concentration [6–8]. The flow rate and components of unstimulated human saliva display diurnal rhythms, with the secretory volume increasing during the day in the active phase and decreasing at night during the resting phase [9]. The concentrations of epithelial growth factor and nerve growth factor [10], as well as adrenergic receptor density [11], also show diurnal rhythms in the SMGs of mice.
Previous studies have shown that aquaporins (AQPs) play an important role in regulating water permeability in salivary secretion. AQPs form a family of membrane water channels that primarily function to transport water across the plasma membrane. To date, 13 members of this family (AQP0–AQP12) have been identified in mammals [12–14], among which AQP1, AQP4, AQP5, and AQP8 are expressed in the salivary glands [15]. Previous studies have demonstrated that pilocarpine stimulation induces a 40% increase in saliva release from AQP5 null mice compared with wild-type (WT) mice, although the secretion of proteins such as amylase was not affected [16, 17]. The AQP5 is one of the major molecules related to saliva secretion, and it has therefore been suggested that AQP5 is associated with the diurnal rhythms of unstimulated saliva secretion.
The central circadian pacemaker, a cluster of hypothalamic neurons located in the suprachiasmatic nucleus (SCN) of the hypothalamus, regulates various physiological functions [18]. Circadian oscillators are also located in other brain regions outside the SCN [19, 20], as well as in peripheral tissues [21–23]. A hierarchical architecture in the circadian system has been proposed in which each peripheral clock oscillates with intrinsic circadian periodicities, with the SCN synchronizing the timing of these peripheral oscillators via neuronal and humoral pathways [24]. Studies have shown that experimental isolation or blockade of the central clock output interrupts circadian rhythms at various levels of physiology, underscoring the importance of the SCN as a circadian pacemaker [25–27].
The circadian oscillations in individual cells are composed of interacting positive and negative transcriptional–translational feedback loops (TTFLs) involving the BMAL1-CLOCK transcriptional activator complex and their target genes which are members of the period (Per1, Per2, Per3) and cryptochrome (Cry1, Cry2) family of circadian clock genes, respectively [24]. The corresponding proteins (PER1, PER2, PER3 and CRY1 and CRY2) act as negative regulators that suppress the activity of BMAL1-CLOCK. Cry1 and Cry2 have been found to be required for normal circadian rhythms: Cry1 −/− Cry2 −/− mice exhibit arrhythmic behavior in constant darkness [28]. In the mouse SMG, expression of clock genes shows circadian variation in vivo [21, 29, 30], suggesting that the SMG has an intrinsic molecular oscillation system. It has also been speculated that this local molecular clock regulates the circadian rhythm of saliva secretion [30].
The aim of the study reported here was to determine whether the circadian expression of Aqp5, an important molecule regulating water permeability in salivary secretion, is driven by the master clock in the SCN or whether it is regulated by the autonomous molecular clock in the SMG. We examined expression levels of the clock genes Per2 and Bmal1, the clock-controlled gene Dbp, and Aqp5 in the SMGs of mice in vivo and compared the expression profiles of these genes with those in isolated SMG cultures ex vivo.
Materials and methods
Ethics statement
This study was carried out in strict accordance with the laws and notifications of the Japanese government. The protocol was approved by the Animal Care and Use Committee at Osaka University (permission# AD-20-042). All tissue sampling was performed with the animals under anesthesia, and all efforts were made to minimize suffering.
Animals
Knock-in mice expressing the PERIOD2::LUCIFERASE fusion protein (PER2::LUC; [23]) and Cry1 −/−, Cry2 −/− and Cry1 −/− Cry2 −/− mice [28] were used in this study. PER2::LUC mice were purchased from the Jackson Laboratory (Strain#006852; Bar Harbor, ME USA). Cry1 +/− and Cry2 +/−mice were backcrossed with the C57BL/6J Jms Slc strain for ten generations and bred with PER2::LUC mice carrying a PER2 fusion luciferase reporter. These were intercrossed to generate Cry1 −/−and Cry2 −/− mice with a heterozygous Per2::Luc gene [20]. Animals were housed in groups and kept under a light regimen of 12 h of light and 12 h of darkness [lights on at 0800 hours; lights off at 2000 hours; lights off was defined as Zeitgeber time (ZT) 12]. Food and water were available ad libitum.
Bright-field and bioluminescence imaging
Male 3-month-old heterozygous PER2::LUC mice were euthanized by cervical dislocation at ZT 7. The SMG was rapidly removed, placed in ice-cold Hank's buffered salt solution (HBSS) and then sliced into 50-μm thick sections using a D.S.K LinearSlicer PRO 7 (Dosaka EM Co., Ltd, Kyoto, Japan). SMG slices were placed onto MilliCell-CM Culture Plate Inserts (PICM ORG 50; Millipore, Billerica, MA) in a 35-mm plastic dish (1000-035; IWAKI, Tokyo, Japan) containing 1.2 mL of recording medium [Dulbecco’s Modified Eagle Medium (Life Technologies, Carlsbad, CA) supplemented with 7.5% sodium bicarbonate solution, 10 mM HEPES, 25 U/mL penicillin, 25 μg/mL streptomycin, B27 supplement (Life Technologies), and 0.1 mM luciferin]. Dishes were sealed with a gas-permeable FEP film 25F (0803-18-16-11; TGK Co., Ltd., Tokyo, Japan) using silicone grease (G-40 M; Shin-Etsu Chemical Co., Ltd., Tokyo, Japan). Bright-field images and bioluminescence images were acquired according to previously reported procedures [31]. Briefly, images from SMG slices were captured using an ImagEM Enhanced EM-CCD camera (Hamamatsu Photonics, Hamamatsu, Japan; frame rate 32 frames/s; EM gain 1200; objective lens; UPlanSApo ×10, NA 0.40; Olympus, Tokyo, Japan) in a light-tight 36.0 °C environmental chamber. The bioluminescence images were stored as consecutive 50-s summed images every 1 min for 3 days, with recording starting at ZT 12. Pseudocoloring was applied to PER2::LUC images using ImageJ software (http://imagej.nih.gov/ij/).
Real–time monitoring of bioluminescence in cultured SMGs
Male 2–3 month-old WT, Cry1 −/−, and Cry2 −/− mice carrying the PER2::LUC allele were euthanized by cervical dislocation under anesthesia at ZT 12, and the SMG was rapidly dissected, trimmed to 2-mm thickness, and processed in the same manner as previously described [32]. SMG sections were maintained in the dark in an incubator at 35 °C and continuously monitored with a photomultiplier tube (Hamamatsu Photonics K.K., Shizuoka, Japan) for 7 days (n = 8 mice of each genotype). Bioluminescence data were analyzed as previously described [20, 33] with a slight modification. Briefly, bioluminescence data were determined by detrending (a 24-h moving average was subtracted from the raw data) and smoothing (using a 3-h moving average) of the raw data. In this smoothed data, the acrophase was determined as the highest point that occurred between 18 and 42 h after the start of bioluminescence recording. The period of PER2::LUC activity was calculated by averaging the periods between each consecutive peak from the first four peaks of the smoothed data.
SMG tissue collection at six time points
For in vivo experiments, male and female 2- to 9-month-old WT and Cry1 −/− Cry2 −/− mice were euthanized by cervical dislocation under anesthesia, and SMGs were rapidly dissected at six different time points: ZT 4, 8, 12, 16, 20, and 24 (n = 6 mice per time point for WT and n = 4 mice per time point for Cry1 −/− Cry2 −/−; equal numbers of male and female mice were used for each group). SMG tissue was kept in a freezer at −80 °C with TRIzol reagent (Life Technologies) until later use.
Preparation of SMG cultures at ZT 0 and ZT 12
For ex vivo experiments, male 2–3 month-old PER2::LUC mice were euthanized by cervical dislocation under anesthesia at ZT 0 or 12, and SMGs were rapidly placed in chilled HBSS and dissected into approximately 24 sections. Sections were equally divided into six dishes, which were maintained in the dark in an incubator at 35 °C. Sectioned SMGs were collected at 8, 12, 16, 20, 24, and 28 h after each preparation at ZT 0 or ZT 12 (n = 4–6); collection of samples of the ZT 0 preparation were started at the projected ZT 8 and that of samples of the ZT 12 preparation at the projected ZT 20. SMG tissue was kept in a freezer at −80 °C with TRIzol reagent (Life Technologies) until later use.
RNA isolation and real-time quantitative RT-PCR
Total RNA was isolated from the SMG using TRIzol reagent and a PureLink RNA Mini kit (Life Technologies) and reverse-transcribed using a PrimeScript RT Reagent kit (TaKaRa BIO Inc.; Otsu, Shiga, Japan) according to the manufacturers’ instructions. Quantitative PCR analysis of the individual cDNAs was performed using the iQ SYBR Green Supermix (Bio-Rad, Hercules, CA). PCR cycling was performed for 40 cycles (at 95 °C for 10 s and 55 °C for 30 s) using a MyiQ Single Color Real-Time PCR Detection System (Bio-Rad) and the following PCR primers: Actb (FW: 5′-ctcttttccagccttccttc-3′, RV: 5′-atctccttctgcatcctgtc-3′); Per2 (FW: 5′-ccagaggaactagcctataagaacca-3′, RV: 5′-gaactcgcacttccttttcagg-3′ [26]); Bmal1 (FW: 5′-cagtgccactgactaccaagaaa-3′, RV: 5′-cctcccaagcattcttgatcc-3′ [26]; Dbp (FW: 5′-aggaactgaagcctcaaccaatc-3′, RV: 5′-ctccggctccagtacttctcat-3′ [26]); Aqp5 (FW: 5′-ggccctcttaataggcaacc-3′, RV: 5′-ttgcctggtgttgtgttgtt-3′ [25]).
Quantitative reverse transcriptase (RT)-PCR results were normalized to Actb mRNA levels, and a relative quantification of mRNA was determined using the ΔCT method. The CT value of target genes was subtracted from that of Actb at each time point using the following formula:
2−ΔCT indicated the relative expression level of the target gene mRNA, normalized to the mean value of the mRNA level in WT mice, with levels expressed as the relative intensity at each point.
Statistics
For the bioluminescence analyses, one-way analysis of variance (ANOVA) was used to compare the period length and the first peak phase of PER2::LUC activity in the SMGs of WT, Cry1 −/−, and Cry2 −/− mice. For mRNA expression profile analysis, one-way ANOVA was used to detect the difference in the time-dependent expression levels of each gene.
Results
Localization of PER2::LUC activity in the mouse SMG
To localize the molecular clock component in the mouse SMG, we first obtained bioluminescence images of PER2::LUC activity in this region. Bright-field images revealed the acinus of the SMG and delineated lumens of the associated ducts (Fig. 1a). PER2 expression was strongly detected in acinar cells and showed robust fluctuation in acini. The circadian change in PER2::LUC activity was also observed in an SMG slice (Fig. 1b).
Fig. 1.
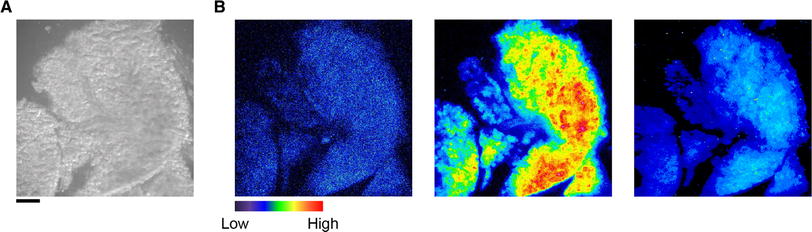
Abundant expression of the PERIOD2::LUCIFERASE fusion protein (PER2::LUC) in the acinus of the submandibular gland (SMG) except for the ductal lumens. a, b Representative bright-field (a) and bioluminescence (b) images at the first trough (left), first peak (middle), and second trough (right) in the SMG of a PER2::LUC mouse. Scale bar 250 μm, pseudocolor bar relative intensity of PER2::LUC activity
Circadian rhythms of PER2::LUC activity in the SMG were associated with mouse genotype
The circadian rhythms of PER2::LUC activity were examined to determine whether the molecular clock also oscillates in cultured SMG ex vivo. PER2::LUC activity in the SMGs of WT and Cry2 −/− mice was robustly rhythmic and persisted for >5 days (Fig. 2a, left and right graphs), while that in the SMGs of Cry1 −/− mice showed a similar circadian rhythmicity but with a more rapid dampening of the rhythm (Fig. 2a, middle graph). The SMG of each genotype had its own characteristic period (Fig. 2b) and phase (Fig. 2c). The average period of Cry1 −/− SMGs [mean ± standard deviation (SD) 20.3 ± 0.4 h) was significantly shorter than that of WT SMGs (23.0 ± 0.8 h), while that of Cry2 −/− SMGs (25.1 ± 0.8 h) was significantly longer (Fig. 2b; one-way ANOVA, p < 0.01). The first peak after culturing was at 29.9 ± 0.8 h in WT SMGs, 23.9 ± 1.2 h in Cry1 −/− SMGs, and 37.9 ± 3.4 h in Cry2 −/− SMGs (Fig. 2c). The differences in peak phase showed statistical significance (one-way ANOVA, p < 0.01).
Fig. 2.
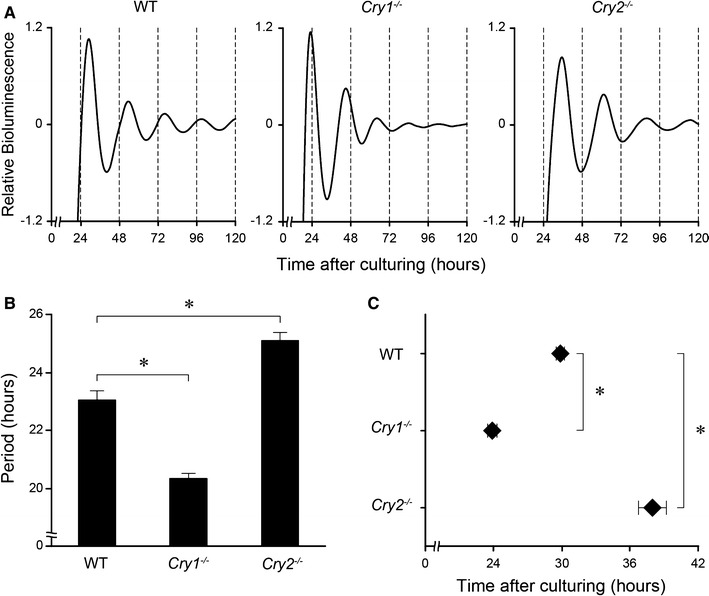
Circadian rhythms in PER2::LUC in cultured SMGs of wild-type (WT), Cry1 −/−, and Cry2 −/− mice ex vivo. a Representative bioluminescence oscillations showing PER2::LUC activity in the SMG of WT (left), Cry1 −/− (middle), and Cry2 −/− (right) mice. Hours, starting from the time when tissues were cultured, are displayed on the X-axis. Serial de-trended bioluminescence counts are plotted for 5 days. b Circadian periods of PER2::LUC rhythms in the SMG of each genotype. c The first peak phase of PER2::LUC rhythms in the SMG of each genotype. All values are the mean ± standard deviation (n = 8 in each genotype). * P < 0.01 (vs. WT, Scheffé post hoc test)
Daily fluctuations in Per2, Bmal1, Dbp, and Aqp5 mRNA levels in the SMG were disrupted in Cry1−/−Cry2−/− mice
To investigate whether the expression of the major clock genes Per2 and Bmal1 and the clock-controlled gene Dbp oscillates in the SMG, we measured the expression levels of these genes throughout the day by quantitative RT-PCR using samples collected in vivo. Because no differences were observed with regard to the sex of the mice, we analyzed data with mixed samples that included equal numbers of male and female mice for each time point. Per2 mRNA in the SMG of WT mice showed diurnal variation (Fig. 3a, left graph; one-way ANOVA, p < 0.001), as did Bmal1 and Dbp mRNA (Fig. 3b, c, left graph; one-way ANOVA, Bmal1: p < 0.001; Dbp: p < 0.001). In contrast, the expression of Per2, Bmal1, and Dbp mRNA in the SMG of Cry1 −/− Cry2 −/− mice did not show significant variations (Fig. 3a–c, right graph; one-way ANOVA, Per2: p = 0.800; Bmal1: p = 0.822; Dbp: p = 0.307). Compared with rhythms in WT mice, the expression levels of Per2 and Dbp mRNA were highly consistent throughout the day in Cry1 −/− Cry2 −/− mice while, in contrast, the expression of Bmal1 mRNA was depressed throughout the day in Cry1 −/− Cry2 −/− mice. We also examined the diurnal variation of Aqp5 mRNA expression in the SMG of WT and Cry1 −/− Cry2 −/− mice in vivo and observed that Aqp5 mRNA expression in the SMG of WT mice showed diurnal variations (Fig. 3d, left graph; one-way ANOVA, p = 0.037), but did not vary in the SMG of Cry1 −/− Cry2 −/− mice (Fig. 3d, right graph; one-way ANOVA, p = 0.994).
Fig. 3.
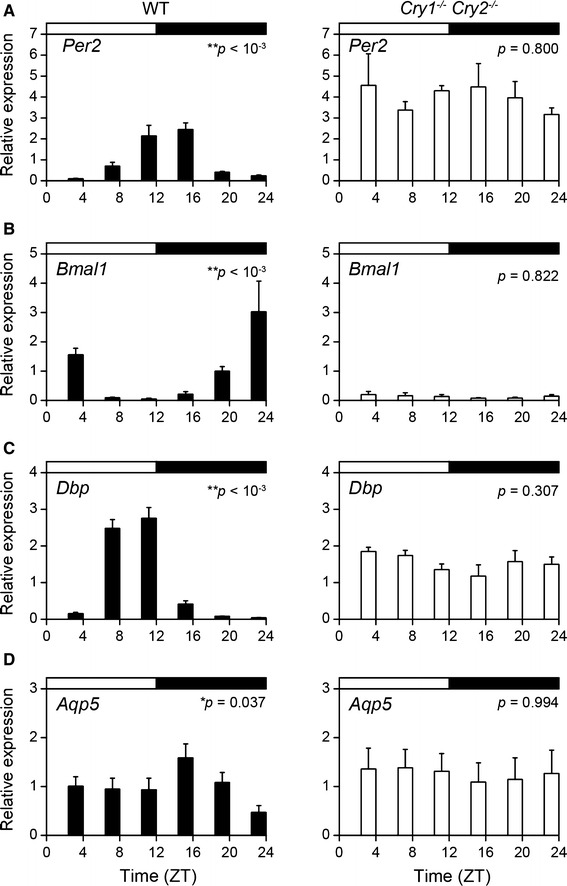
Daily expression profiles of clock genes Per2 and Bmal1, clock-controlled gene Dbp, and aquaporin-5 (Aqp5) mRNA in the SMGs of Cry1 −/− Cry2 −/− mice collected in vivo. a–d Per2 (a), Bmal1 (b), Dbp (c), and Aqp5 (d) mRNA expression levels in the SMGs of WT mice (black columns) and Cry1 −/− Cry2 −/− mice (white columns). The black and white horizontal bar at the top of each graph indicates the dark and light phases, respectively. Data are shown as mean ± standard error of the mean (SEM) (n = 6 WT mice per time point and n = 4 Cry1 −/− Cry2 −/− mice per time point). Significance of the analysis of variance: *p < 0.05, **p < 0.01. ZT Zeitgeber time
Circadian rhythms were detected in the expression of Per2, Bmal1, and Dbp mRNA, but not in that of Aqp5 mRNA in cultured SMGs
The SMGs of WT mice were cultured and the tissue collected at projected ZT 0, 4, 8, 12, 16 and 20. Expression of Per2, Bmal1, and Dbp mRNA showed circadian rhythm-associated variations (Fig. 4a–c; one-way ANOVA, Per2: p < 0.001; Bmal1: p < 0.001; Dbp: p < 0.001). The expression profiles of Aqp5 mRNA from ZT 0 and ZT 12 preparations showed a two-peak variation along a 24-h time course (Fig. 4d). However, individual plots did not show any circadian fluctuations although they revealed a steady shrinking over time regardless of preparation time (Fig. 4e, f; one-way ANOVA, ZT 0: p < 0.001; ZT 12: p < 0.001).
Fig. 4.
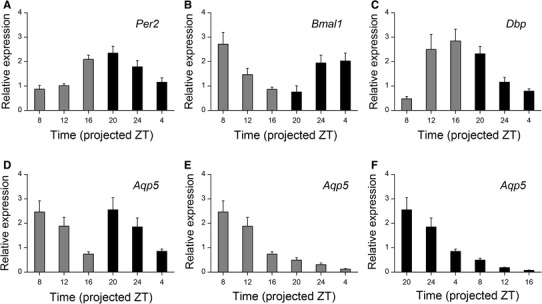
Circadian expression profiles of Per2, Bmal1, Dbp, and Aqp5 mRNA in cultured SMGs ex vivo. a–c Per2 (a), Bmal1 (b), and Dbp (c) mRNA expression levels in SMG cultures prepared at ZT 0 (gray column) and ZT 12 (black column). Samples at ZT 8, 12, and 16 were collected from a ZT 0 preparation, and samples from ZT 20, 24, and 4 were collected from a ZT 12 preparation. d–f Aqp5 mRNA expression levels in samples (d) prepared at ZT 0 (e) and ZT 12 (f). Samples were collected at the projected ZTs described on each chart. Data are shown as mean ± SEM (n = 5 mice per time point in ZT 0 preparation and n = 6 mice per time point in ZT 12 preparation)
Discussion
We observed that the rhythms of the major clock genes Per2 and Bmal1 and the clock-controlled gene Dbp oscillate in SMGs collected both in vivo and ex vivo in a manner dependent on the expression of Cry1 and Cry2. These results were confirmed by bioluminescence recordings of PER2::LUC activity. Moreover, the circadian and diurnal rhythms of these clock genes were associated with the phenotype of Cry1 −/−, Cry2 −/−, and Cry1 −/− Cry2 −/− mice. These data support the hypothesis that the SMG has self-sustaining oscillators driven by the molecular clock. We also observed rhythmic expression of Aqp5 mRNA in vivo, but not ex vivo. The observation that the circadian rhythmicity of Aqp5 mRNA expression disappeared in cultured SMGs suggests that its rhythm within the SMG is predominantly driven by the master clock in the SCN via several pathways. These potentially include the circadian rhythms in the autonomic nervous system governed by the SCN [34] and indirectly through feeding rhythms that accompany metabolic changes and mechanical stimuli [21].
In mammals, the existence of a tissue-autonomous peripheral clock was proposed based on the circadian rhythm in the biological response of mouse adrenal glands ex vivo [35]. Cultured hamster retinas that exhibited circadian rhythms of melatonin synthesis in vitro with periods dependent on circadian genotypes provided direct evidence for these peripheral clocks [36]. Cry1 −/− and Cry2 −/− mice exhibit a shorter and longer free-running period, respectively, than WT mice [28]. Furthermore, Cry1 −/− Cry2 −/− mice, which lack a circadian clock, show arrhythmic behavior, physiology, and metabolism [28, 37]. In the present study, we examined PER2::LUC rhythms in the SMG of WT, Cry1 −/−, and Cry2 −/− mice and compared period lengths. We observed that the circadian rhythms in the SMG were altered in Cry1 −/− and Cry2 −/− mice. Compared with WT mice, the period length was shorter in Cry1 −/− mice, while the period was longer in Cry2 −/− mice. These data are consistent with the behavioral phenotypes of Cry1 −/− and Cry2 −/− mice, as well as with the results of a previous study that described PER2::LUC rhythms in SCN explants from Cry1 −/− and Cry2 −/− mice [20, 38]. We observed robust PER2::LUC activity in the acini of the SMG. The localization of the expression of clock genes was consistent with a previous study that reported the expression of these genes in the three major types of salivary glands [30]. Furthermore, previous studies demonstrated that AQP5 expression is localized in acinar cells in human, rat, and mouse salivary glands. These findings suggest that salivation is related to the molecular clock composed of clock genes [15, 39, 40].
The mRNA expression profiles of Per2, Bmal1, and Dbp from in vivo experiments in the SMG showed daily rhythms. The rhythms of Per2 and Bmal1 mRNA expression were anti-phase with respect to each other. In contrast, the expression profiles in the SMGs of Cry1 −/− Cry2 −/− mice did not show rhythmicity, with Per2 mRNA expressed at consistently high levels and Bmal1 mRNA expressed at consistently low levels throughout the day. These results are consistent with a previous study examining those expression profiles in the adrenal glands of Cry1 −/− Cry2 −/− mice [37]. Furthermore, we examined the expression profiles of Per2 and Bmal1 in cultured SMGs ex vivo, which were isolated from, and not affected by, autonomic innervation or variations in body temperature and feeding. In the ex vivo experiments, the mRNA expression profiles of Per2 and Bmal1 also showed circadian rhythms and were anti-phase with respect to each other. These results indicate that cultured SMGs remained healthy throughout the sampling course of our study. Dbp is a clock-controlled gene containing an E-box motif in the promoter region, and its expression shows circadian rhythms through E-box regulation [41]. In the present study, expression of Dbp mRNA in the SMGs of WT mice showed circadian rhythms in samples collected both in vivo and ex vivo. In contrast, in the SMGs of Cry1 −/− Cry2 −/− mice, Dbp mRNA was expressed at consistently high levels throughout the day. These results reveal that the expression of Dbp in the SMG is dependent on E-box regulation. Taken together, these results suggest that the SMG has self-sustaining oscillators driven by TTFLs.
Previous studies have demonstrated that AQP5 plays an important role in water permeability in the salivary glands [16, 42]. In our study, expression of Aqp5 mRNA in the SMG of WT mice showed significant daily variation in vivo, and its acrophase was observed at midnight (ZT 16) during the active phase in mice. In the SMGs of Cry1 −/− Cry2 −/− mice, Aqp5 mRNA was expressed at intermediate levels throughout the day. These results indicate that the expression of Aqp5 is affected by the circadian system. However, Aqp5 mRNA did not show any circadian fluctuation in ex vivo experiments, indicating that in cultured SMGs, expression of Aqp5 mRNA was no longer predominantly regulated by cell-autonomous oscillations. Therefore, exhaustive analysis in salivary glands may provide the important information for the circadian regulation of Aqp5 in vivo [43]. In contrast to the decline of Aqp5 rhythmicity in cultured SMGs, in vitro circadian rhythms were reported for melatonin synthesis in the retina [36] and reactivity to adrenocorticotropic hormone in the adrenal glands [35], which could be under the control of cell-autonomous oscillations. In a study conducted in sympathectomized rats, the phase of Per1 expression in denervated SMG entrained robustly to the feeding schedule, although with an intact SMG, the phase mostly entrained to light-dark cycle information via the autonomic nervous system [44]. On the other hand, the results from a previous study suggest that the circadian variation in mucin secretion from respiratory submucosal glands is under the control of the SCN via vagal nerve [45]. Taken together, these results indicate that the daily rhythm of Aqp5 expression is predominantly controlled by the central clock in the SCN via the autonomic pathway, feeding, and other entrainment factors under normal conditions, whereas denervated and/or isolated conditions, such as ex vivo culture conditions, lead to a decline in rhythmicity.
In summary, our study demonstrates that the mouse SMG contains self-sustaining circadian oscillators driven by TTFLs. Although the circadian expression of Aqp5 has been proposed to be directly regulated by TTFLs [30], our ex vivo data suggest that it is predominantly driven by the master clock in the SCN via the autonomic nervous system. Further studies are necessary to elucidate the regulatory mechanisms that control the circadian expression of functional molecules in the SMG.
Author contributions
H.U. and W.N. designed the research; H.U., T.J.N., N.N.T., and W.N. performed the research; A.O.K., H.O., T.T., and T.S. contributed new reagents/analytic tools; H.U., T.J.N., N.N.T., and W.N. analyzed the data; H.U., T.J.N., N.N.T., and W.N. wrote the paper.
Compliance with ethical standards
Conflict of interest
The authors declare that they have no conflict of interest.
Funding
This work was supported by JSPS KAKENHI grant numbers 26462809, 26860160, 26861780. N.N.T. is a research fellow of the Japan Society for the Promotion of Science.
Ethical approval
All applicable international, national, and/or institutional guidelines for the care and use of animals were followed. The Protocol was approved by the Animal Care and Use Committee at Osaka University.
References
- 1.Brosky ME. The role of saliva in oral health: strategies for prevention and management of xerostomia. J Support Oncol. 2007;5:215–225. [PubMed] [Google Scholar]
- 2.Rudney JD. Does variability in salivary protein concentrations influence oral microbial ecology and oral health? Crit Rev Oral Biol Med. 1995;6:343–367. doi: 10.1177/10454411950060040501. [DOI] [PubMed] [Google Scholar]
- 3.Cohen S. Isolation of a mouse submaxillary gland protein accelerating incisor eruption and eyelid opening in the new-born animal. J Biol Chem. 1962;237:1555–1562. [PubMed] [Google Scholar]
- 4.Levine MJ. Development of artificial salivas. Crit Rev Oral Biol Med. 1993;4:279–286. doi: 10.1177/10454411930040030401. [DOI] [PubMed] [Google Scholar]
- 5.Schneyer LH, Levin LK. Rate of secretion by individual salivary gland pairs of man under conditions of reduced exogenous stimulation. J Appl Physiol. 1955;7:508–512. doi: 10.1152/jappl.1955.7.5.508. [DOI] [PubMed] [Google Scholar]
- 6.Abe K, Dawes C. The effects of electrical and pharmacological stimulation on the types of proteins secreted by rat parotid and submandibular glands. Arch Oral Biol. 1978;23:367–372. doi: 10.1016/0003-9969(78)90094-8. [DOI] [PubMed] [Google Scholar]
- 7.Garrett JR, Suleiman AM, Anderson LC, Proctor GB. Secretory responses in granular ducts and acini of submandibular glands in vivo to parasympathetic or sympathetic nerve stimulation in rats. Cell Tissue Res. 1991;264:117–126. doi: 10.1007/BF00305729. [DOI] [PubMed] [Google Scholar]
- 8.Garrett JR, Thulin A. Changes in parotid acinar cells accompanying salivary secretion in rats on sympathetic or parasympathetic nerve stimulation. Cell Tissue Res. 1975;159:179–193. doi: 10.1007/BF00219154. [DOI] [PubMed] [Google Scholar]
- 9.Dawes C. Circadian rhythms in human salivary flow rate and composition. J Physiol. 1972;220:529–545. doi: 10.1113/jphysiol.1972.sp009721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Siminoski K, Bernanke J, Murphy RA. Nerve growth factor and epidermal growth factor in mouse submandibular glands: identical diurnal changes and rates of secretagogue-induced synthesis. Endocrinology. 1993;132:2031–2037. doi: 10.1210/endo.132.5.8477654. [DOI] [PubMed] [Google Scholar]
- 11.Basso A, Piantanelli L. Influence of age on circadian rhythms of adrenoceptors in brain cortex, heart and submandibular glands of BALB/c mice: when circadian studies are not only useful but necessary. Exp Gerontol. 2002;37:1441–1450. doi: 10.1016/S0531-5565(02)00126-2. [DOI] [PubMed] [Google Scholar]
- 12.Agre P, King LS, Yasui M, Guggino WB, Ottersen OP, Fujiyoshi Y, Engel A, Nielsen S. Aquaporin water channels–from atomic structure to clinical medicine. J Physiol. 2002;542:3–16. doi: 10.1113/jphysiol.2002.020818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Castle NA. Aquaporins as targets for drug discovery. Drug Discov Today. 2005;10:485–493. doi: 10.1016/S1359-6446(05)03390-8. [DOI] [PubMed] [Google Scholar]
- 14.Verkman AS. More than just water channels: unexpected cellular roles of aquaporins. J Cell Sci. 2005;118:3225–3232. doi: 10.1242/jcs.02519. [DOI] [PubMed] [Google Scholar]
- 15.Delporte C, Steinfeld S. Distribution and roles of aquaporins in salivary glands. Biochim Biophys Acta. 2006;1758:1061–1070. doi: 10.1016/j.bbamem.2006.01.022. [DOI] [PubMed] [Google Scholar]
- 16.Ma T, Song Y, Gillespie A, Carlson EJ, Epstein CJ, Verkman AS. Defective secretion of saliva in transgenic mice lacking aquaporin-5 water channels. J Biol Chem. 1999;274:20071–20074. doi: 10.1074/jbc.274.29.20071. [DOI] [PubMed] [Google Scholar]
- 17.Yang B, Song Y, Zhao D, Verkman AS. Phenotype analysis of aquaporin-8 null mice. Am J Physiol Cell Physiol. 2005;288:C1161–C1170. doi: 10.1152/ajpcell.00564.2004. [DOI] [PubMed] [Google Scholar]
- 18.Nakamura TJ, Takasu NN, Nakamura W. The suprachiasmatic nucleus: age-related decline in biological rhythms. J Physiol Sci. 2016;66:367–374. doi: 10.1007/s12576-016-0439-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Abe M, Herzog ED, Yamazaki S, Straume M, Tei H, Sakaki Y, Menaker M, Block GD. Circadian rhythms in isolated brain regions. J Neurosci. 2002;22:350–356. doi: 10.1523/JNEUROSCI.22-01-00350.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Uchida H, Nakamura TJ, Takasu NN, Todo T, Sakai T, Nakamura W. Cryptochrome-dependent circadian periods in the arcuate nucleus. Neurosci Lett. 2016;610:123–128. doi: 10.1016/j.neulet.2015.10.071. [DOI] [PubMed] [Google Scholar]
- 21.Tahara Y, Kuroda H, Saito K, Nakajima Y, Kubo Y, Ohnishi N, Seo Y, Otsuka M, Fuse Y, Ohura Y, et al. In vivo monitoring of peripheral circadian clocks in the mouse. Curr Biol. 2012;22:1029–1034. doi: 10.1016/j.cub.2012.04.009. [DOI] [PubMed] [Google Scholar]
- 22.Yamazaki S, Numano R, Abe M, Hida A, Takahashi R, Ueda M, Block GD, Sakaki Y, Menaker M, Tei H. Resetting central and peripheral circadian oscillators in transgenic rats. Science. 2000;288:682–685. doi: 10.1126/science.288.5466.682. [DOI] [PubMed] [Google Scholar]
- 23.Yoo SH, Yamazaki S, Lowrey PL, Shimomura K, Ko CH, Buhr ED, Siepka SM, Hong HK, Oh WJ, Yoo OJ, et al. PERIOD2::LUCIFERASE real-time reporting of circadian dynamics reveals persistent circadian oscillations in mouse peripheral tissues. Proc Natl Acad Sci USA. 2004;101:5339–5346. doi: 10.1073/pnas.0308709101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Reppert SM, Weaver DR. Coordination of circadian timing in mammals. Nature. 2002;418:935–941. doi: 10.1038/nature00965. [DOI] [PubMed] [Google Scholar]
- 25.Inouye ST, Kawamura H. Persistence of circadian rhythmicity in a mammalian hypothalamic “island” containing the suprachiasmatic nucleus. Proc Natl Acad Sci USA. 1979;76:5962–5966. doi: 10.1073/pnas.76.11.5962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Meyer-Bernstein EL, Jetton AE, Matsumoto SI, Markuns JF, Lehman MN, Bittman EL. Effects of suprachiasmatic transplants on circadian rhythms of neuroendocrine function in golden hamsters. Endocrinology. 1999;140:207–218. doi: 10.1210/endo.140.1.6428. [DOI] [PubMed] [Google Scholar]
- 27.Schwartz WJ, Gross RA, Morton MT. The suprachiasmatic nuclei contain a tetrodotoxin-resistant circadian pacemaker. Proc Natl Acad Sci USA. 1987;84:1694–1698. doi: 10.1073/pnas.84.6.1694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Vitaterna MH, Selby CP, Todo T, Niwa H, Thompson C, Fruechte EM, Hitomi K, Thresher RJ, Ishikawa T, Miyazaki J, et al. Differential regulation of mammalian period genes and circadian rhythmicity by cryptochromes 1 and 2. Proc Natl Acad Sci USA. 1999;96:12114–12119. doi: 10.1073/pnas.96.21.12114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Furukawa M, Kawamoto T, Noshiro M, Honda KK, Sakai M, Fujimoto K, Honma S, Honma K, Hamada T, Kato Y. Clock gene expression in the submandibular glands. J Dent Res. 2005;84:1193–1197. doi: 10.1177/154405910508401219. [DOI] [PubMed] [Google Scholar]
- 30.Zheng L, Seon YJ, McHugh J, Papagerakis S, Papagerakis P. Clock genes show circadian rhythms in salivary glands. J Dent Res. 2012;91:783–788. doi: 10.1177/0022034512451450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Nakamura TJ, Nakamura W, Tokuda IT, Ishikawa T, Kudo T, Colwell CS, Block GD (2015) Age-related changes in the circadian system unmasked by constant conditions. eNeuro 2(4). doi:10.1523/ENEURO.0064-15.2015 [DOI] [PMC free article] [PubMed]
- 32.Sakai T, Larsen M, Yamada KM. Fibronectin requirement in branching morphogenesis. Nature. 2003;423:876–881. doi: 10.1038/nature01712. [DOI] [PubMed] [Google Scholar]
- 33.Nakamura W, Yamazaki S, Takasu NN, Mishima K, Block GD. Differential response of Period 1 expression within the suprachiasmatic nucleus. J Neurosci. 2005;25:5481–5487. doi: 10.1523/JNEUROSCI.0889-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Ueyama T, Krout KE, Nguyen XV, Karpitskiy V, Kollert A, Mettenleiter TC, Loewy AD. Suprachiasmatic nucleus: a central autonomic clock. Nat Neurosci. 1999;2:1051–1053. doi: 10.1038/15973. [DOI] [PubMed] [Google Scholar]
- 35.Ungar F, Halberg F. Circadian rhythm in the in vitro response of mouse adrenal to adrenocorticotropic hormone. Science. 1962;137:1058–1060. doi: 10.1126/science.137.3535.1058. [DOI] [PubMed] [Google Scholar]
- 36.Tosini G, Menaker M. Circadian rhythms in cultured mammalian retina. Science. 1996;272:419–421. doi: 10.1126/science.272.5260.419. [DOI] [PubMed] [Google Scholar]
- 37.Doi M, Takahashi Y, Komatsu R, Yamazaki F, Yamada H, Haraguchi S, Emoto N, Okuno Y, Tsujimoto G, Kanematsu A, et al. Salt-sensitive hypertension in circadian clock-deficient Cry-null mice involves dysregulated adrenal Hsd3b6. Nat Med. 2010;16:67–74. doi: 10.1038/nm.2061. [DOI] [PubMed] [Google Scholar]
- 38.Evans JA, Pan H, Liu AC, Welsh DK. Cry1−/− circadian rhythmicity depends on SCN intercellular coupling. J Biol Rhythms. 2012;27:443–452. doi: 10.1177/0748730412461246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Gresz V, Kwon TH, Hurley PT, Varga G, Zelles T, Nielsen S, Case RM, Steward MC. Identification and localization of aquaporin water channels in human salivary glands. Am J Physiol Gastrointest Liver Physiol. 2001;281:G247–G254. doi: 10.1152/ajpgi.2001.281.1.G247. [DOI] [PubMed] [Google Scholar]
- 40.Matsuzaki T, Ablimit A, Suzuki T, Aoki T, Hagiwara H, Takata K. Changes of aquaporin 5-distribution during release and reaccumulation of secretory granules in isoproterenol-treated mouse parotid gland. J Electron Microsc (Tokyo) 2006;55:183–189. doi: 10.1093/jmicro/dfl023. [DOI] [PubMed] [Google Scholar]
- 41.Ueda HR, Hayashi S, Chen W, Sano M, Machida M, Shigeyoshi Y, Iino M, Hashimoto S. System-level identification of transcriptional circuits underlying mammalian circadian clocks. Nat Genet. 2005;37:187–192. doi: 10.1038/ng1504. [DOI] [PubMed] [Google Scholar]
- 42.Krane CM, Melvin JE, Nguyen HV, Richardson L, Towne JE, Doetschman T, Menon AG. Salivary acinar cells from aquaporin 5-deficient mice have decreased membrane water permeability and altered cell volume regulation. J Biol Chem. 2001;276:23413–23420. doi: 10.1074/jbc.M008760200. [DOI] [PubMed] [Google Scholar]
- 43.Koike N, Yoo SH, Huang HC, Kumar V, Lee C, Kim TK, Takahashi JS. Transcriptional architecture and chromatin landscape of the core circadian clock in mammals. Science. 2012;338:349–354. doi: 10.1126/science.1226339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Vujovic N, Davidson AJ, Menaker M. Sympathetic input modulates, but does not determine, phase of peripheral circadian oscillators. Am J Physiol Regul Integr Comp Physiol. 2008;295:R355–R360. doi: 10.1152/ajpregu.00498.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Bnado H, Nishio T, van der Horst GTJ, Masubuchi S, Hisa Y, Okamura H. Vagal regulation of respiratory clocks in mice. J Neurosci. 2007;27:4359–4365. doi: 10.1523/JNEUROSCI.4131-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]


