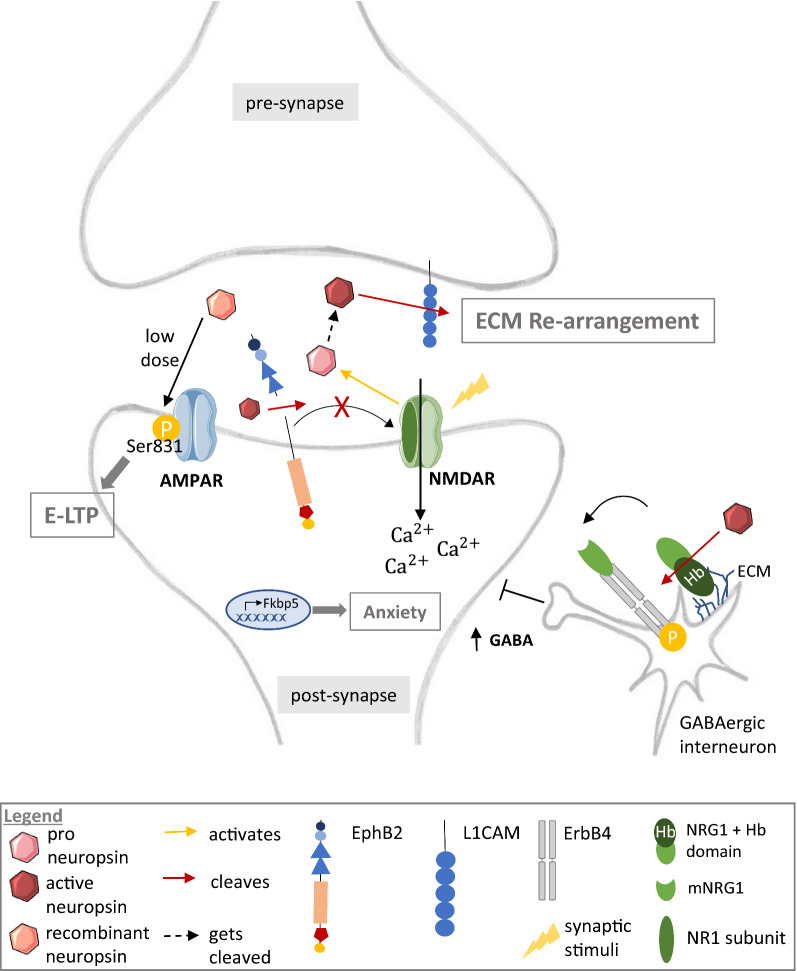
Fig. 4.
Neuropsin pathways implicated in synaptic plasticity. When an action potential arrives at the pre-synapse, this signal will be transmitted to the post-synapse by activating the NMDA receptor (NMDAR). NMDAR activation leads to the removal of the activity-masking peptide of proneuropsin (light red) and results in neuropsin activation. Furthermore, stress induces an increased Klk8 expression. Active neuropsin (dark red) then cleaves (red arrow) its substrate L1CAM (blue), EphB2 (peach/blue) and NRG1 (green). L1CAM cleavage in the hippocampus might lead to an increased flexibility of synaptic structures. Extracellular cleavage of EphB2 in the amygdala prevents EphB2 and NMDAR clustering and hereby enhances the NMDAR current. This induces Fkbp5 gene expression and behavioral signs of anxiety. After removal of the heparin binding domain (dark green, Hb) mature NRG1 (mNRG1, light green) binds to its receptor ErbB4 (grey) in GABAergic interneurons. Consequently, ErbB4 (grey) gets phosphorylated (yellow circle). ErbB4 phosphorylation is critical for GABAergic inhibitory transmission. Thus neuropsin-dependent NRG1 cleavage modulates E-LTP through the modulation of inhibitory projections into CA1 pyramidal cells in the hippocampus. Recombinant neuropsin furthermore leads to phosphorylation of the AMPA receptor (AMPAR) at a phosphorylation site specific for E-LTP (Ser831). Elements downloaded from [41]