Abstract
The objective of this study was to explore the role of apelin in the healing of gastric lesions induced by stress. Male Wistar rats were exposed to water immersion and restraint stress (WIRS) for 6 h with or without the apelin receptor antagonist F13A. The rats were killed on the 1st, 3rd, 5th or 10th day after the end of stress induction. Apelin and hypoxia-inducible factor-1α expression was increased on the 1st day after the end of stress exposure and was decreased daily thereafter. However, F13A retarded the healing of gastric lesions by preventing the improvement of mucosal blood flow, prostaglandin E2 production and vascular endothelial growth factor expression in rats exposed to WIRS. Additionally, F13A increased the gastric 4-hydroxynonenol + malondialdehyde content on the 1st and 3rd days after the end of stress induction but did not affect the change in gastric mucosal nitric oxide levels. In conclusion, apelin may be a regulatory protein involved in the healing mechanism of stress-induced gastric damage.
Keywords: Apelin, F13A, Gastric mucosa, Lesion, Water immersion and restraint stress
Introduction
Apelin is the endogenous ligand for the G protein-linked orphan receptor APJ [1]. Preproapelin consists of 77 amino acids and is cleaved into several molecular forms in different tissues, including apelin-36, apelin-17 and apelin-13 [2]. A variety of physiological functions for apelin have been described, including neuroprotection, regulation of cardiovascular function, immune response, water intake and feeding behavior [2, 3].
Apelin and the APJ receptor are widely expressed throughout the body [2, 4], and both apelin and APJ are highly expressed in the gastrointestinal system, especially in the stomach [4, 5]. Apelin mRNA has been detected by immunohistochemical methods in gastric mucous neck, parietal, chief and endocrine cells [6], and APJ immunostaining has been shown on the surface epithelium of the rat stomach [4, 5]. The distribution of the apelin/APJ system in the stomach indicates a potential role for apelin in gastric functions. Apelin is an angiogenic factor that is involved in the proliferation of gastric cells [4] and the regulation of gastric acid secretion [5, 7, 8]. Recently, centrally administered apelin-13 was shown to inhibit gastric emptying in mice [9].
The stomach is one of the main targets of stress. Gastric ulceration caused by stress is an example of a stress-induced organ injury. Water immersion and restraint stress (WIRS), which is a complex of physical and psychological stressors, causes lesions that mimic the gastric lesions caused by sepsis, trauma or surgery [10, 11]. The gastric mucosal lesions induced by stress are associated with increased gastric acid secretion, nitric oxide production and oxidative stress as well as decreased gastric mucosal prostaglandin production and gastric blood flow [12]. The recovery period of gastric mucosal damage is a dynamic process that involves inflammation, cell proliferation and differentiation, an increase in the gastric blood flow at the ulcer margin, the expression of cytokines and growth factors and the formation of new vessels at the ulcer bed [13, 14]. The major stimuli for the gastric mucosal lesion-healing process are growth factors, such as vascular endothelial growth factor (VEGF) and epidermal growth factor (EGF) and vasodilator mediators, such as prostaglandin E2 (PGE2) and nitric oxide (NO). These factors are produced at the injury site and activate epithelial cell migration and proliferation via autocrine and/or paracrine effects [15, 16].
The regulatory mechanism underlying apelin expression remains unknown; however, our previous findings indicated that gastric apelin expression was upregulated by WIRS and that the increase in apelin expression in the gastric mucosa of rats exposed to WIRS might be due to the induction of hypoxia-inducible factor-1 α (HIF-1α). According to our study, the apelin-mediated reduction in lipid peroxidation in the gastric mucosa can be regarded as a protective effect against gastric damage due to stress [17]. However, the role of apelin in gastrointestinal physiology has received little attention, and the effects of apelin on the healing of gastric damage have not been previously described.
The aim of this study was to determine the role of apelin in the healing of gastric lesions induced by WIRS. The WIRS model appears to be very suitable to test the effect of various factors on the healing of gastric mucosal lesions [10, 17]. In the present study, we evaluated the gastric mucosal blood flow (GMBF), which is a component of the gastric mucosal barrier, and accompanying changes in PGE2 and NO production in the gastric mucosa. Oxidative stress was assessed by measuring the mucosal malondialdehyde (MDA) plus 4-hydroxynonenal (4-HNE) content. Additionally, mucosal proliferative and angiogenic factors, including apelin and VEGF, were analyzed on the 1st, 3rd, 5th and 10th days after the end of stress exposure. Finally, the APJ receptor antagonist F13A was used to determine whether apelin was involved in gastric injury healing.
Materials and methods
Animals
The 180 adult male Wistar rats weighing 250–300 g used in this study were provided by the Akdeniz University Faculty of Medicine Experimental Animals Care and Production Unit. The rats were housed in a temperature-controlled environment (23 ± 1 °C) on a 12-h light/dark cycle with free access to rat chow and tap water. The rats were allowed to acclimate to the conditions for at least 7 days prior to the experiments. All experimental procedures were performed in accordance with mandated standards of human care and were approved by the Animal Care and Use Committee of Akdeniz University.
Experimental protocol
The animals were randomly divided into nine groups (8–10 rats per group) as follows: control group, the rats were not exposed to stress; WIRS 1 day group, the rats were killed on the 1st day after the end of stress induction; WIRS 3 day group, the rats were killed on the 3rd day after the end of stress induction; WIRS 5 day group, the rats were killed on the 5th day after the end of stress induction; WIRS 10 day group, the rats were killed on the 10th day after the end of stress induction; F13A + WIRS 1 day group, the rats were treated with the APJ receptor antagonist F13A immediately prior to WIRS and killed on the 1st day after the end of stress induction; F13A + WIRS 3 day group, the rats were treated with F13A immediately prior to WIRS and killed on the 3rd day after the end of stress induction; F13A + WIRS 5 day group, the rats were treated with F13A immediately prior to WIRS and killed on the 5th day after the end of stress induction; F13A + WIRS 10 day group, the rats were treated with F13A immediately prior to WIRS and killed on the 10th day after the end of stress induction.
In the WIRS and F13A + WIRS groups, the conscious rats were restrained individually in rectangular polypropylene cages (28 cm high × 8 cm wide × 8 cm deep) and immersed up to the depth of the xyphoid process in a 23 °C water bath for 6 h to induce WIRS, as described in previous reports [10, 17, 18]. F13A (150 µg/kg, i.v., Phoenix Pharmaceuticals, Burlingame, CA, USA, 057-29) was injected into the tail veins of the rats in the F13A + WIRS groups immediately prior to the application of WIRS. Following an 18-h fasting period, the animals were anesthetized with a single intraperitoneal (i.p.) injection of xylazine-ketamine (10 and 90 mg/kg, respectively, Alfasan International B.V., Woerden, The Netherlands). After measurement of the GMBF, stomach specimens were collected for analysis.
Western blotting analysis of apelin and VEGF
Tissues were homogenized in 300 μl of lysis buffer [10 mM Tris (pH 7.4), 100 mM NaCl, 1 mM EDTA, 1 mM EGTA, 1 mM NaF, 20 mM Na4P2O7, 2 mM Na3VO4, 1 % Triton-X, 10 % glycerol, 0.1 % SDS and 0.5 % deoxycholate] supplemented with 5 μl of a protease inhibitor cocktail (Sigma-Aldrich, St. Louis, MO, USA, P2714) per 100 mg of gastric tissue. The samples were centrifuged at 15,000 rpm for 10 min at 4 °C. Then, the supernatants were mixed with reagents such as the NuPAGE LDS sample buffer (4X) (Invitrogen, Carlsbad, CA, USA, NP0007) and reducing agent (10X) (Invitrogen, Carlsbad, CA, USA, NP0004). The protein concentration was determined with a commercial kit (Thermo Scientific, Rockford, IL, USA, NJ176939) according to the method of Bradford et al. [19].
Prior to electrophoresis, the samples were boiled for 10 min at 70 °C. Samples containing 35 μg of total protein were loaded onto a 10 % NuPAGE-Bis-Tris Gel (Invitrogen, Carlsbad, CA, USA, NP0316BOX) with a molecular weight marker (Fermentas, LT-02241 Vilnius, Lithuania, SM1811). The gels were run at 200 mV for 45 min (XCell SureLock™ Mini-Cell Electrophoresis System, Carlsbad, CA, USA, EI0001), and then, the proteins were transferred onto a PVDF membrane for 7 min (iBlot® Dry Blotting System, Invitrogen, Carlsbad, CA, USA, IB1001).
The membrane was processed for Western blotting analysis (BenchPro Card Processing Station, Invitrogen, Carlsbad, CA, USA, 4100) using the following steps: 30 min of blocking (5 % non-fat dry milk in 10 mM PBS, pH 7.4); 10 min rinse (double-distilled water); 60 min incubation with the apelin (sc-33805) or VEGF (sc-507) primary antibody (Santa Cruz Biotechnology, CA, USA) diluted 1:200 in 5 % non-fat dry milk in 10 mM PBS (pH 7.4); 20 min wash (10 mM PBS, pH 7.4); 30 min incubation with an HRP-conjugated secondary antibody [Santa Cruz Biotechnology, CA, USA (sc-2004)] diluted 1:4000 in 5 % non-fat dry milk in 10 mM PBS (pH 7.4); 20 min wash; 6 min rinse. After stripping, the same procedure was performed using a GAPDH antibody (Santa Cruz Biotechnology, CA, USA, sc-47724, diluted 1:250) in BenchPro. Finally, the blots were visualized using a chemiluminescent detection system kit according to the manufacturer’s instructions (Chemicon, Temecula, CA, USA, 2600). The membranes were exposed to hyperfilm (Amersham Biosciences, Buckinghamshire, UK, RPN3103 K) and subsequently analyzed using the ImageJ v. 1.37 software [20].
Immunohistochemistry
To detect HIF-1α and apelin expression using immunohistochemistry, 5-μm-thick sections of formalin-fixed paraffin-embedded tissues were dried overnight at 56 °C. The samples were deparaffinized twice in xylene at room temperature for 10 min, dehydrated with a graded ethanol series and then washed in distilled water. Antigen retrieval was performed by soaking the slides in a citrate buffer (pH 6.0, 0.01 M) and then boiling them in a microwave oven at 100 °C for 7 min. Endogenous peroxidase activity was blocked by incubating the sections with 0.3 % H2O2 for 15 min. Then, the slides were incubated with blocking serum for 7 min to block non-specific immunoglobulin binding. The sections were incubated with either an anti-HIF-1α mouse monoclonal primary antibody (Santa Cruz Biotechnology, CA, USA, sc-53546) or an anti-apelin rabbit monoclonal primary antibody (Santa Cruz Biotechnology, CA, USA, sc-33805) at a 1:400 dilution overnight at 4 °C. Subsequently, the sections were incubated with a biotinylated goat anti-mouse IgG (Vector, Burlingame, CA, USA, BA-9200) for HIF-1α or a horseradish peroxidase (HRP)-conjugated secondary antibody (Santa Cruz Biotechnology, CA, USA, sc-2004) for apelin at a 1:400 dilution for 45 min. Then, the sections were overlaid with peroxidase-labeled streptavidin (HRP LSAB-2 system, K0609, DakoCytomation, Glostrup, Denmark) for 25 min, followed by rinsing in PBS for 15 min. Diaminobenzidine (DAB, Vector, Burlingame, CA, USA) was added as a substrate, and the sections were observed until they simultaneously turned brown. The evaluation of the immunohistochemical labeling of HIF-1α in the samples was performed using H-SCORE [21]. Briefly, the sections were evaluated using an Axioplan microscope (Zeiss, Oberkochen, Germany) with a special ocular scale. Three randomly selected slides, each representing five different fields at a 200× magnification, were evaluated for the immunochemical labeling of HIF-1α. The labeling was scored in a semi-quantitative fashion that included the intensity of the specific labeling in the sections. For each tissue, an H-SCORE value was derived by summing the percentages of cells labeled at each intensity multiplied by the weighted intensity of the labeling as follows: H-SCORE = ∑ P i (i + 1), where i is the intensity score and P i is the corresponding percentage of cells. Two observers blinded to the experimental groups performed the H-SCORE evaluations, and the average score was used.
Gastric lesion evaluation
To determine the lesion index, the stomachs were removed, incised along the greater curvature and pinned onto a platform. The macroscopic appearance of the stomachs was imaged and transferred to a computer. The total hemorrhagic erosions were determined as the lesion index (in mm2) using the ‘SPOT Advanced Analysis Program’.
Gastric mucosal blood flow measurement
The gastric mucosal blood flow was assessed using laser Doppler flowmetry (LDF 100C, Model TSD 145, Biopac, Goleta, CA, USA), which is based on the principle of light reflectance from moving red blood cells [22]. The rats were anesthetized with xylazine-ketamine, and their abdomens were opened with a midline incision. The stomach was exposed and carefully opened along the greater curvature. The gastric content was gently evacuated, and the gastric mucosa was washed with 5 ml of PBS (pH 7.4). An optical probe immobilized using a micromanipulator was gently placed 0.5 mm above perpendicular to the mucosal surface in the oxyntic gland area to monitor the GMBF, which was displayed in mV (value of the Doppler signal voltage) on the digital panel of the flowmeter. After stabilization, four points were selected for measurement (one point for 1 min), and the average value was calculated as the BPU (blood per unit).
Determination of the PGE2 concentration
The PGE2 assay was performed according to the protocol supplied with the PGE2 Enzyme Immunoassay Kit (Cayman, Ann Arbor, MI, USA, 514010). At the end of the experimental period, the stomachs were opened along the greater curvature. The mucosa was scraped with glass slides, immediately frozen in liquid nitrogen and stored at −80 °C. During analysis, the tissue scrapings were weighed and homogenized in 1 ml of homogenization buffer (0.1 M phosphate buffer, pH 7.4) containing 1 mM EDTA and 10 µM indomethacin per 100 mg of tissue. Then, the tissue homogenates were spun at 8000×g for 10 min. According to the protocol, the resultant supernatants were diluted 1:500 with assay buffer. After measurement of the protein concentration, the PGE2 level was assayed in 96-well plates. The PGE2 level in the gastric mucosa is expressed as pg per mg of tissue.
Determination of the nitrate/nitrite concentration
The nitrate/nitrite (NOx) concentration in the stomach was determined using a colorimetric assay kit (Cayman, Ann Arbor, MI, USA, 780001). Briefly, tissue samples were weighed and homogenized in 1 ml of PBS (pH 7.4) per 100 mg of tissue. The exudates were centrifuged at 2500 rpm for 10 min. The supernatant was passed through a 30 kDa filter by centrifugation at 29,000×g for 60 min. The filtrate was incubated with the nitrate reductase and cofactor mixtures for 3 h at room temperature according to the manufacturer’s instructions to convert the nitrate in the sample to nitrite. The optical density of the wells was measured with a microplate reader (Biotek, ELx800, Highland Park, VT, USA) at 540 nm, and the total nitrite concentration, which reflected the amount of NOx in the sample, was calculated using a standard nitrate curve after reaction with Griess reagent. The results are expressed as µM per mg of protein.
Determination of 4-HNE-MDA concentration
To evaluate lipid peroxidation in the gastric mucosa, the 4-HNE plus MDA concentration was determined according to the protocol supplied with the colorimetric assay (Oxis Research Bioxytech LPO 586, Burlingame, CA, USA, 21012). Briefly, tissue samples were homogenized in 10 ml of ice-cold phosphate buffered saline (PBS, 20 mM, pH 7.4) per 1 g of tissue. Prior to homogenization, 10 µl of 0.5 M butylated hydroxytoluene in acetonitrile was added to 1 ml of tissue homogenate to prevent the oxidation of the tissue sample during homogenization. The prepared homogenate was centrifuged at 3000×g at 4 °C for 10 min. The resultant clean supernatant was used for the assay. The samples were transferred to a colorimetric assay kit according to the manufacturer’s instructions. The 4-HNE plus MDA concentration was calculated using a standard curve, and the results were expressed as nM per mg of tissue.
Statistical analysis
The data were analyzed using the SPSS v. 13.0 software. The Kruskal-Wallis test followed by the Mann-Whitney U test was used to evaluate the data. Quantitative data were described as medians (minimum-maximum values). For the graphical presentation of the medians (minimum-maximum values), box plots [display showing the upper and the lower quartiles (75 and 25 percentiles, respectively) and range] were used. P < 0.05 was considered statistically significant.
Results
Stress-induced apelin expression gradually diminishes during the healing of gastric mucosal lesions
To assess the relationship between the apelin expression and the healing of gastric lesions induced by stress, we determined the changes of apelin expression via Western blotting analysis during gastric lesion healing. Increased apelin expression was observed on the 1st day after the end of WIRS induction [0.99 (0.74–1.10) in the control group and 2.33 (2.06–3.25) in the WIRS 1 day group, p < 0.05]. However, the apelin overexpression gradually diminished in the days following the stress exposure and returned to the level of the control group on the 10th day after the end of stress induction (p < 0.05 vs. the WIRS 1 day group) (Fig. 1a).
Fig. 1.
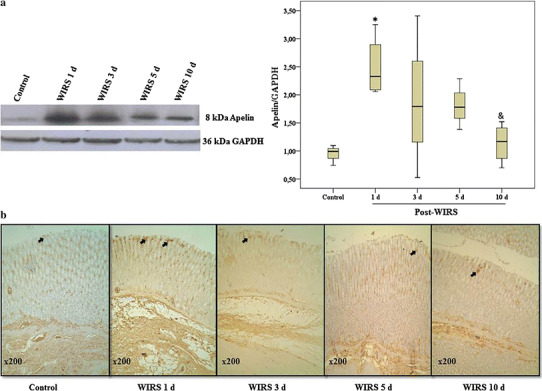
a Determination of apelin expression in gastric tissues by Western blotting analysis during gastric lesion healing in rats exposed to WIRS for 6 h. Data are presented as box plots showing the range, quartiles (25–75 %) and median. *p < 0.05 vs. the control group; & p < 0.05 vs. the WIRS 1 day group. b Immunohistochemical staining of apelin in the gastric samples during gastric lesion healing in rats exposed to WIRS for 6 h. Original magnification ×200 (n = 4 rats/group)
The immunohistochemical analysis demonstrated an increase in apelin staining in the neck region of the gastric mucosa after exposure to stress (Fig. 1b).
Stress-induced HIF-1α expression diminishes during the healing of gastric mucosal lesions
To assess the relationship between HIF-1α expression and the healing of gastric lesions induced by stress, we determined the changes in HIF-1α expression via immunohistochemical analysis during gastric lesion healing. Similar to apelin expression, increased HIF-1α expression was observed on the 1st day after the end of stress induction (p < 0.05 vs. the control group) and gradually diminished during gastric mucosal lesion healing (Fig. 2).
Fig. 2.
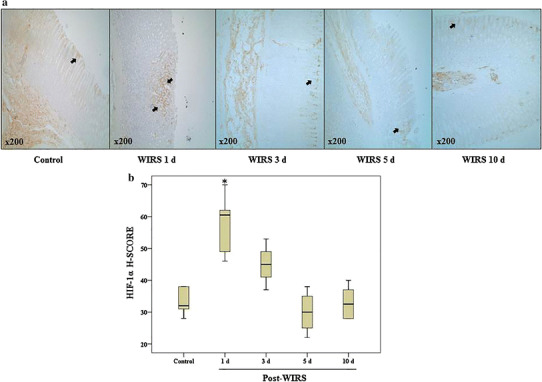
a Immunohistochemical labeling of HIF-1α in gastric tissues during gastric lesion healing in rats exposed to WIRS for 6 h. Original magnification ×200. b Graphics of the mathematical values of the H-SCORE evaluations of HIF-1α in gastric tissues. Data are presented as box plots showing the range, quartiles (25–75 %) and median. *p < 0.05 vs. the control group (n = 6 rats/group)
The APJ receptor antagonist retards the healing of gastric mucosal lesions
As shown in Fig. 3a, b, marked hemorrhagic lesions in the gastric mucosa were observed on the 1st day after the end of stress induction (p < 0.01 vs. the control group). Although hemorrhagic lesions were still observed on the 3rd and 5th days after the end of WIRS induction, the lesions were almost completely healed on the 10th day after the end of stress induction. However, F13A treatment delayed gastric lesion healing, with the gastric lesions significantly increased on the 5th and 10th days after the end of the stress exposure (p < 0.05 vs. WIRS 5 day group and p < 0.05 vs. WIRS 10 day group).
Fig. 3.
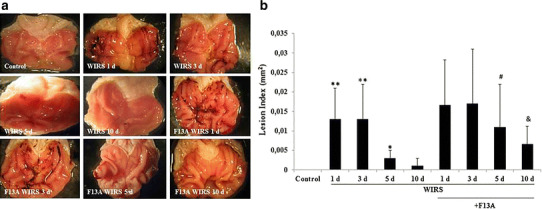
Gastric lesion index in stressed rats treated with and without F13A (150 µg/kg b.w., i.v.) during gastric lesion healing on the 1st, 3rd, 5th or 10th day after the end of stress induction. a Representative macroscopic appearance of gastric tissues of the experimental rats. b Lesion index. Data are presented as box plots showing the range, quartiles (25–75 %) and median. **p < 0.01 vs. the control group, # p < 0.05 vs. the WIRS 5 day group, & p < 0.05 vs. the WIRS 10 day group (n = 6 rats/group)
The APJ receptor antagonist prevents the recovery of gastric mucosal blood flow during gastric mucosal lesion healing
As shown in Fig. 4, the mucosal blood flow was 338.65 (298.80–362.30) BPU in the intact gastric mucosa. The GMBF was decreased on the 1st, 3rd and 5th days after the end of stress induction (p < 0.01 vs. the control group) and then returned to the value of the control group on the 10th day after the end of stress induction. F13A pretreatment (150 μg/kg b.w., i.v.) did not change the GMBF in the early phase of gastric damage healing. However, F13A pretreatment prevented the increase in GMBF on the 10th day after the end of stress induction (p < 0.001 vs. the WIRS 10 day group).
Fig. 4.
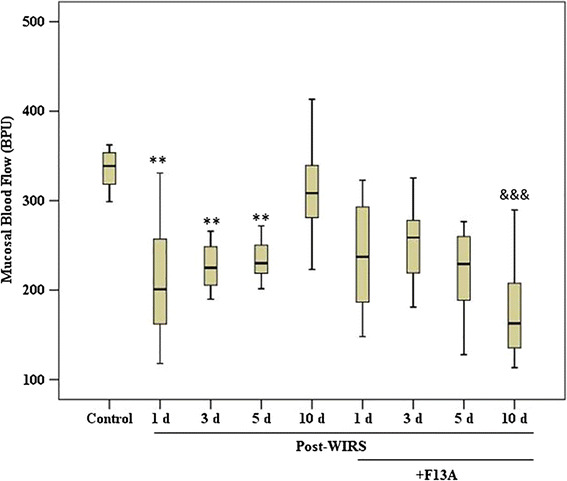
Gastric mucosal blood flow in stressed rats treated with and without F13A (150 µg/kg b.w., i.v.) during gastric lesion healing on the 1st, 3rd, 5th or 10th day after the end of stress induction. Data are presented as box plots showing the range, quartiles (25–75 %) and median. **p < 0.01 vs. the control group; &&& p < 0.001 vs. the WIRS 10 day group (n = 8–10 rats/group)
The APJ receptor antagonist prevents the increase in VEGF production during the late phase of gastric mucosal lesion healing
During the stress-induced gastric lesion-healing process, VEGF expression was increased on the 3rd day (p < 0.05 vs. the control group) and remained high on the 5th and 10th days after the end of WIRS (p < 0.05 vs. the control group). However, blockade of the APJ receptor reduced VEGF expression in the gastric tissues on the 5th and 10th days after the end of WIRS (p < 0.05 vs. the WIRS 5 day group and p < 0.05 vs. the WIRS 10 day group) (Fig. 5).
Fig. 5.
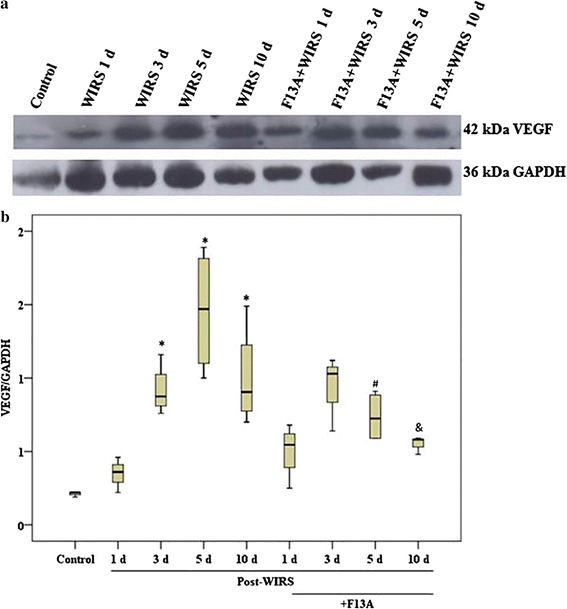
VEGF expression in gastric mucosa samples obtained from the stressed rats treated with and without F13A (150 µg/kg b.w., i.v.) during gastric lesion healing on the 1st, 3rd, 5th or 10th day after the end of stress induction. a Representative appearance of VEGF expression in the gastric samples of the experimental groups. b Graphic of the mathematical values of the VEGF/GAPDH ratios in the gastric samples. Data are presented as box plots showing the range, quartiles (25–75 %) and median. *p < 0.05 vs. the control group, # p < 0.05 vs. the WIRS 5 day group and & p < 0.05 vs. the WIRS 10 day group) (n = 4 rats/group)
The APJ receptor antagonist prevents the increase in PGE2 production during gastric mucosal lesion healing
As shown in Fig. 6, the gastric PGE2 content was 233 (42–356) pg/mg tissue in the control rats. The PGE2 level was decreased to 126 (88–259) pg/mg tissue on the 1st day after the end of stress exposure. On the 5th and 10th days after the end of WIRS exposure, which corresponded to the late phase of the healing process, the gastric PGE2 concentration was significantly increased compared with the control group [336 (125–527) pg/mg tissue in the WIRS 5 day group and 320 (268–560) pg/mg tissue in the WIRS 10 day group, p < 0.05]. However, pretreatment with F13A significantly prevented the increase in the gastric PGE2 concentration on the 10th day after WIRS exposure [272 (189–307) pg/mg tissue, p < 0.05 vs. the WIRS 10 day group].
Fig. 6.
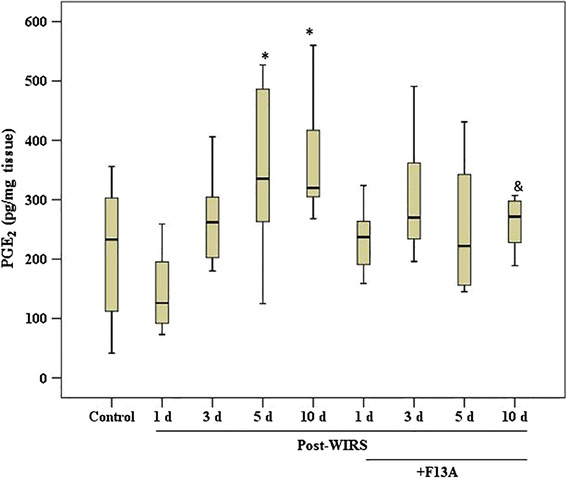
PGE2 concentration in the gastric mucosa samples obtained from the stressed rats treated with and without F13A (150 µg/kg b.w., i.v.) during gastric lesion healing on the 1st, 3rd, 5th or 10th day after the end of stress induction. Data are presented as box plots showing the range, quartiles (25–75 %) and median. *p < 0.05 vs. the control group and & p < 0.05 vs. the WIRS 10 day group (n = 8–10 rats/group)
The APJ receptor antagonist does not affect NOx production during gastric mucosal lesion healing
As shown in Fig. 7, the gastric NOx concentration was 84.9 (33.6–109.6) μM/mg protein in the control rats. The gastric NOx content was increased to 162.4 (140.0–230.6) in the WIRS 3 day group (p < 0.05 vs. the control group), 144.3 (50.2–224.6) in the WIRS 5 day group (p < 0.05 vs. the control group) and 170.4 (79–316) μM/mg protein in the WIRS 10 day group (p < 0.05 vs. the control group). F13A treatment did not affect gastric NOx production in rats exposed to WIRS.
Fig. 7.
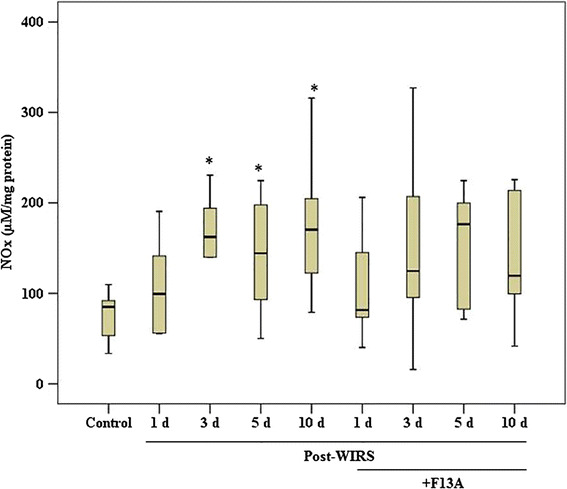
Nitrate/nitrite (NOx) concentration in the gastric mucosa samples obtained from the stressed rats treated with and without F13A (150 µg/kg b.w., i.v.) during gastric lesion healing on the 1st, 3rd, 5th or 10th day after the end of stress induction. Data are presented as box plots showing the range, quartiles (25–75 %) and median. *p < 0.05 vs. the control group (n = 8–10 rats/group)
The APJ receptor antagonist increases lipid peroxidation during the early phase of gastric mucosal lesion healing
Figure 8 shows the effect of the APJ receptor antagonist on lipid peroxidation during the gastric lesion-healing process. Here, the 4-HNE plus MDA concentrations in the gastric mucosa were increased in rats exposed to WIRS. During gastric lesion healing, the gastric mucosal 4-HNE plus MDA concentrations were increased 2.5-fold on the 1st day (p < 0.01 vs. the control group), 2.7-fold on the 3rd day (p < 0.001 vs. the control group), 5.2-fold on the 5th day (p < 0.001 vs. the control group), and 5.7-fold on the 10th day (p < 0.001 vs. the control group). Inhibition of the effect of apelin using F13A increased the gastric mucosal 4-HNE plus MDA concentrations on the 1st day (p < 0.05 vs. the WIRS 1 day) and the 3rd day after the end of stress induction (p < 0.001 vs. the WIRS 3 day). However, the lipid peroxidation product concentrations were unchanged in response to F13A treatment on the 5th and 10th days after the end of stress induction.
Fig. 8.
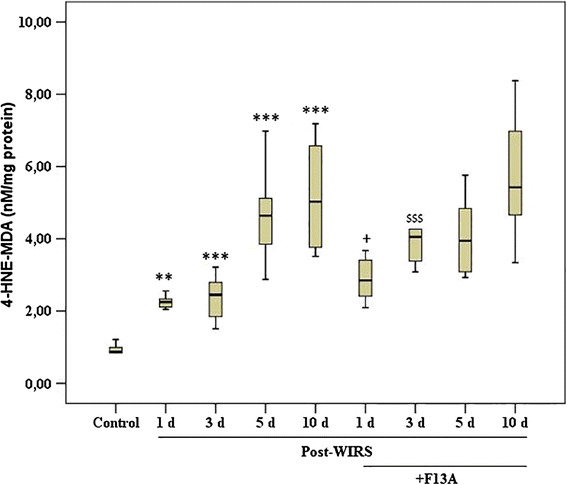
The changes in lipid peroxidation products (4HNE + MDA) in stomachs obtained from the stressed rats treated with and without F13A (150 µg/kg b.w., i.v.) during gastric lesion healing on the 1st, 3rd, 5th or 10th day after the end of stress induction. Data are presented as box plots showing the range, quartiles (25–75 %) and median. **p < 0.01 and ***p < 0.001 vs. the control group, + p < 0.05 vs. the WIRS 1 day group and $$$ p < 0.001 vs. the WIRS 3 day group (n = 8–10 rats/group)
Discussion
Our current study focuses on the mucosal expression of apelin during the recovery phase of gastric damage after the end of WIRS exposure and the effects of endogenous apelin on the rate of healing of WIRS-induced gastric damage. Previous studies conducted using experimental stress models emphasized the importance of NO and PGE2, their interaction with VEGF and the products of lipid peroxidation in the healing of WIRS-induced gastric lesions [16, 23, 24]. This study provides evidence that inhibition of the effects of apelin using F13A retards the healing of acute gastric lesions. This effect of F13A is accompanied by a marked increase in the level of lipid peroxides and a decrease in PGE2 production and VEGF expression in the gastric mucosa. These effects, accompanied by the disappearance of the increase in the GMBF, suggest that apelin accelerates the healing of stress-induced gastric lesions. This study demonstrates for the first time the beneficial effect of apelin on the healing of WIRS-induced gastric lesions.
Using Western blotting analysis, we detected apelin overexpression during the recovery period of gastric mucosal damage induced by WIRS. Our present immunohistochemical analyses demonstrated a time-dependent increase in apelin staining in the neck region of the gastric mucosa after stress exposure. Maximal apelin expression was observed on the 1st day after the end of exposure to stress and decreased gradually over the following days. The increased apelin staining was similar to the findings obtained from the Western blotting analysis. Although the regulation of apelin expression remains uncharacterized, previous studies have demonstrated that apelin gene expression may be controlled by the oxygen level and HIF-1α [25, 26]. Our results indicate that the increase in apelin expression is accompanied by an increase in HIF-1α expression. Based on our current knowledge, stress causes both sympathetic and parasympathetic stimulation of the stomach, thereby increasing gastric motility and muscular contraction. These changes ultimately lead to vascular compression and mucosal ischemia. Sympathetic stimulation also causes arteriolar vasoconstriction and thus reduces the blood flow to the gastric mucosa, leading to local hypoxia and ischemia [27]. HIF-1α is activated under hypoxic conditions because of the inhibition of oxygen-dependent HIF-1α prolyl-4-hydroxylase-mediated proline hydroxylation under normoxic conditions, which targets the HIF-1α subunit for ubiquitination and proteasomal degradation. Additionally, reactive oxygen species (ROS) act as signaling molecules in gastric tissues that stimulate HIF-1α protein synthesis via the activation of the phosphatidylinositol 3′-kinase (PI3 K)/Akt and p42/p44 mitogen-activated protein kinase (MAPK) pathways [28]. The findings of our study suggest that the HIF-1α overexpression induced by WIRS may increase apelin expression.
In our previous study, we investigated the preventive effect of apelin on the formation of stress-induced lesions. The lesion index was measured immediately after the end of stress exposure, and apelin was found to be very effective in preventing gastric lesions in rats exposed to stress [17]. In the present study, the gastric lesion areas induced by stress were smaller than the lesions determined in the stomach in our previous study as a result of the initiation of healing on day 1 after the end of stress. Using an experimental model of stress injury, we found that these lesions were substantially healed within 5 days and almost completely healed within 10 days. Additionally, a decrease in GMBF was noted on the 1st day after the end of stress induction. The GMBF progressively increased, reaching values similar to those recorded for the intact mucosa within 10 days. F13A pretreatment reduced the healing of stress-induced gastric lesions and delayed GMBF recovery. These results indicate that the promoting effect of apelin on the healing of stress-induced gastric lesions is related to the mucosal blood flow during the late phase of healing. The prevention of the increase in the GMBF during the late phase of gastric healing via the apelin blockade suggests that the effect of apelin on the GMBF may be related to angiogenesis. The increase in VEGF expression is followed by an increase in apelin expression, whereas the disappearance of the increase in VEGF expression due to pretreatment with the APJ receptor antagonist is associated with a delay in gastric healing. The present findings indicate that this peptide may be involved in gastric lesion healing on the 3rd day after the end of stress induction. Furthermore, the prevention of VEGF expression with the APJ receptor antagonist may delay the healing of stress-induced gastric lesions.
VEGF, which is a fundamental regulator of angiogenesis, is an essential component of healing [23, 29]. VEGF binds to VEGF-R1 and VEGF-R2 and induces phosphorylation of the cytosolic proteins involved in signal transduction that initiate the proliferation and migration of endothelial cells and microvascular tube formation [16]. Angiogenesis is important because this process provides the damaged area with nutrients and oxygen supplies. Restoration of the blood supply to the damaged area is an important factor in the healing process based on the finding of a correlation between reduced mucosal blood flow and the development of gastric ulcers [14]. Angiogenesis and VEGF play major roles in the healing of gastric ulceration resulting from damaging factors in the gastric mucosal barrier [29]. The stimulation of angiogenesis by VEGF treatment promotes the healing of gastric ulcers in rats [29]. VEGF production is stimulated by nitric oxide and prostaglandins in addition to growth factors and cytokines [16]. Prostaglandins are important vasodilators, and the increased GMBF during the healing of stress-induced damage is related to an enhanced production of prostaglandins as a result of increased COX-1 and COX-2 activity [15, 24]. Prostaglandins appear to play an important role in the repair of the gastric mucosa after acute damage caused by stress via their antisecretory, mucoprotective and hyperemic activities. NSAIDs retard ulcer healing by inhibiting epithelial cell proliferation, migration and angiogenesis [24]. In our study, VEGF expression was markedly observed in the gastric tissue as expected on the 5th day after the formation of WIRS-induced gastric damage. Similarly, PGE2 was simultaneously observed in the stress-induced damaged mucosa. F13A pretreatment prevented the increase in VEGF expression and PGE2 production during the healing process of damaged gastric mucosa. Our findings demonstrated that the enhancing effect of apelin on GMBF was accompanied by VEGF expression and an increase in PGE2 production in damaged gastric mucosa. The gastric blood flow gradually improved during healing and was accompanied by PGE2 production. This finding suggests that endogenous prostaglandins may contribute to VEGF expression and angiogenesis; prostaglandins may also account for the gradual repair of the mucosa after damage induced by WIRS.
NO is an important vasodilator mediator in the healing of stress-induced gastric lesions. NO increases GMBF and accelerates the healing of these lesions [5]. Our findings demonstrated increased NO production in the gastric mucosa during the gastric lesion healing process. Previous studies reported the importance of NO derived from iNOS activity in an ulcer-healing mechanism [30]. The selective suppression of iNOS expression and activity was accompanied by a decrease in NO production, resulting in delayed ulcer healing [14, 30]. Maximal iNOS expression in the inflammatory cells at the ulcer base and ulcer margin was noted on the 3rd day after ulcer induction and declined by day 7 [30, 31]. We demonstrated an increase in NO generation during the healing of lesions induced by stress; however, the blockade of apelin did not affect the NO content of the gastric mucosa. Previous studies suggested that the hypotensive effect induced by apelin/APJ signaling was mediated through NO production via eNOS activation [6, 31]. This finding potentially indicates that apelin is ineffective regarding the production of NO induced by iNOS during the recovery from stress-induced gastric lesions.
Stress-induced gastric lesions are the manifestation of an inflammatory response. Gastric inflammation increases leukocyte adherence to the endothelial surface of post-capillary venules and is characterized by the migration of macrophages and polymorphonuclear leukocytes into the damaged area [12, 24, 32]. Superoxide radical anions produced by neutrophils in the gastric mucosa react with cellular lipids and lead to the breakdown of polyunsaturated fatty acids to reactive short chain aldehydes, such as 4-HNE and MDA [33, 34]. Tissue MDA and 4-HNE levels are typically useful for the assessment of the biological effects of ROS [23, 33]. The MDA plus 4-HNE content of the gastric mucosa is an indicator of lipid peroxidation [35]. We reported that lipid peroxidation was involved in the formation of stress-induced gastric mucosal damage [35, 36]. In our previous study, the protective effect of apelin against stress-induced gastric lesions was associated with its antioxidant effect against lipid peroxidation in the gastric mucosa [17]. Similar to the results of our previous report [17], Foussal et al. showed that apelin stimulated the activity of antioxidant enzymes, such as catalase and superoxide dismutase, in cardiomyocytes and reduced oxidative stress in these cells [37]. Additionally, apelin was recently shown to suppress the production and release of ROS in adipocytes through its interaction with the APJ receptor [38, 39]. According to the findings of Than et al., apelin promotes the expression of antioxidant enzymes via the MAPK kinase/extracellular signal-regulated kinase (ERK) and AMP-activated protein kinase (AMPK) pathways and inhibits the expression of prooxidant enzymes via the AMPK pathway [38]. Pisarenko et al. demonstrated the potential effects of structural apelin analogs on mitochondrial ROS generation in cardiomyocyte apoptosis [39]. In this study, we demonstrated that blocking the APJ receptor led to a marked increase in the formation of lipid peroxides in the early phase of gastric damage healing (the 1st and 3rd days after the end of stress exposure) and that apelin did not show a beneficial effect during the same period of stress-induced gastric lesion healing. Our present study provides evidence that blocking the effect of apelin with F13A delays the healing of WIRS-induced gastric lesions in the late phase of the healing process (the 5th and 10th days after stress). The promoting effect of apelin in the same period of gastric damage healing was associated with increased VEGF expression and PGE2 production in the gastric mucosa. Gastric damage caused by stress begins to heal on the 1st day after the end of stress induction. Various mechanisms are related to the healing of the damaged gastric mucosa. According to our results, the reducing effect of apelin on the production of lipid peroxides in the early phase of gastric damage healing does not play a role during the same gastric damage healing period.
In conclusion, our present results demonstrate a beneficial role for stress-induced apelin during the healing of stress-induced gastric lesions via a mechanism involving the restoration of microcirculation by increasing gastric mucosal PGE2 production and VEGF expression. Further studies may reveal whether apelin is useful for the treatment of various gastrointestinal diseases under stress conditions.
Acknowledgments
This work was supported by The Scientific and Technological Research Council of Turkey (TUBITAK) funded by the Turkish Government (Project number: 110S447).
Abbreviations
- APJ
G protein-linked orphan receptor
- WIRS
Water immersion and restraint stress
- F13A
Apelin receptor antagonist
- GMBF
Gastric mucosal blood flow
- VEGF
Vascular endothelial growth factor
- EGF
Epidermal growth factor
- PGE2
Prostaglandin E2
- NO
Nitric oxide
- HIF-1α
Hypoxia-inducible factor
- MDA
Malondialdehyde
- 4-HNE
4-Hydroxynonenal
Compliance with ethical standards
Conflict of interest
The authors report no conflicts of interest.
References
- 1.O’Carroll AM, Lolait SJ, Harris LE, Pope GR. The apelin receptor APJ: journey from an orphan to a multifaceted regulator of homeostasis. J Endocrinol. 2013;219(1):R13–R35. doi: 10.1530/JOE-13-0227. [DOI] [PubMed] [Google Scholar]
- 2.Ladeiras-Lopes R, Ferreira-Martins J, Leite-Moreira AF. The apelinergic system: the role played in human physiology and pathology and potential therapeutic applications. Arq Bras Cardiol. 2008;90(5):343–349. doi: 10.1590/S0066-782X2008000500012. [DOI] [PubMed] [Google Scholar]
- 3.Barnes G, Japp AG, Newby DE. Translational promise of the apelin–APJ system. Heart. 2010;96(13):1011–1016. doi: 10.1136/hrt.2009.191122. [DOI] [PubMed] [Google Scholar]
- 4.Wang G, Kundu R, Han S, Qi X, Englander EW, Quertermous T, Greeley GH. Ontogeny of apelin and its receptor in the rodent gastrointestinal tract. Regul Pept. 2009;158(1–3):32–39. doi: 10.1016/j.regpep.2009.07.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Wang G, Anini Y, Wei W, Qi X, O’Carroll AM, Mochizuki T, Wang HQ, Hellmich MR, Englander EW, Greeley Jr GH. Apelin, a new enteric peptide: localization in the gastrointestinal tract, ontogeny, and stimulation of gastric cell proliferation and of cholecystokinin secretion. Endocrinology. 2004;145(3):1342–1348. doi: 10.1210/en.2003-1116. [DOI] [PubMed] [Google Scholar]
- 6.Ishida J, Hashimoto T, Hashimoto Y, Nishiwaki S, Iguchi T, Harada S, Sugaya T, Matsuzaki H, Yamamoto R, Shiota N, Okunishi H, Kihara M, Umemura S, Sugiyama F, Yagami K, Kasuya Y, Mochizuki N, Fukamizu A. Regulatory roles for APJ, a seven-transmembrane receptor related to angiotensin-type 1 receptor in blood pressure in vivo. J Biol Chem. 2004;279(25):26274–26279. doi: 10.1074/jbc.M404149200. [DOI] [PubMed] [Google Scholar]
- 7.Chu S, Schubert ML. Gastric secretion. Curr Opin Gastroenterol. 2012;28(6):587–593. doi: 10.1097/MOG.0b013e328358e5cc. [DOI] [PubMed] [Google Scholar]
- 8.Lambrecht NW, Yakubov I, Zer C, Sachs G. Transcriptomes of purified gastric ECL and parietal cells: identification of a novel pathway regulating acid secretion. Physiol Genom. 2006;25(1):153–165. doi: 10.1152/physiolgenomics.00271.2005. [DOI] [PubMed] [Google Scholar]
- 9.Lv SY, Yang YJ, Qin YJ, Xiong W, Chen Q. Effect of centrally administered apelin-13 on gastric emptying and gastrointestinal transit in mice. Peptides. 2011;32(5):978–982. doi: 10.1016/j.peptides.2011.01.023. [DOI] [PubMed] [Google Scholar]
- 10.Guo S, Gao Q, Jiao Q, Hao W, Gao X, Cao JM. Gastric mucosal damage in water immersion stress: mechanism and prevention with GHRP-6. World J Gastroenterol. 2012;18(24):3145–3155. doi: 10.3748/wjg.v18.i24.3145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Lu CL, Li ZP, Zhu JP, Zhao DQ, Ai HB. Studies on functional connections between the supraoptic nucleus and the stomach in rats. J Physiol Sci. 2011;61(3):191–199. doi: 10.1007/s12576-011-0137-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Tan R, Bulbul M, Ongut G, Tosun O, Izgut-Uysal VN. Prostaglandins, capsaicin-sensitive sensory nerves and neutrophil infiltration, but not nitric oxide, contribute to cold restraint stress-induced gastric adaptation in rats. Clin Exp Pharmacol Physiol. 2006;33(10):946–951. doi: 10.1111/j.1440-1681.2006.04469.x. [DOI] [PubMed] [Google Scholar]
- 13.Malara B, Josko J, Tyrpien M, Malara P, Steplewska K. Dynamics of changes in vascular endothelial growth factor (VEGF) expression and angiogenesis in stress-induced gastric ulceration in rats. J Physiol Pharmacol. 2005;56(2):259–271. [PubMed] [Google Scholar]
- 14.Brzozowska I, Ptak-Belowska A, Pawlik M, Pajdo R, Drozdowicz D, Konturek SJ, Pawlik WW, Brzozowski T. Mucosal strengthening activity of central and peripheral melatonin in the mechanism of gastric defense. J Physiol Pharmacol. 2009;60(Suppl 7):47–56. [PubMed] [Google Scholar]
- 15.Hatazawa R, Tanaka A, Tanigami M, Amagase K, Kato S, Ashida Y, Takeuchi K. Cyclooxygenase-2/prostaglandin E2 accelerates the healing of gastric ulcers via EP4 receptors. Am J Physiol Gastrointest Liver Physiol. 2007;293(4):G788–G797. doi: 10.1152/ajpgi.00131.2007. [DOI] [PubMed] [Google Scholar]
- 16.Tarnawski AS. Cellular and molecular mechanisms of gastrointestinal ulcer healing. Dig Dis Sci. 2005;50(Suppl 1):S24–S33. doi: 10.1007/s10620-005-2803-6. [DOI] [PubMed] [Google Scholar]
- 17.Izgut-Uysal VN, Gemici B, Birsen I, Acar N, Ustunel I. The protective effect of apelin against water-immersion and restraint stress-induced gastric damage. J Physiol Sci. 2014;64(4):279–289. doi: 10.1007/s12576-014-0317-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Warzecha Z, Dembinski A, Ceranowicz P, Dembinski M, Cieszkowski J, Kownacki P, Konturek PC. Role of sensory nerves in gastroprotective effect of anandamide in rats. J Physiol Pharmacol. 2011;62(2):207–217. [PubMed] [Google Scholar]
- 19.Bradford MM. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976;72:248–254. doi: 10.1016/0003-2697(76)90527-3. [DOI] [PubMed] [Google Scholar]
- 20.Blackmore MS, Lord CC. The relationship between size and fecundity in Aedes albopictus . J Vector Ecol. 2000;25:2212–2217. [PubMed] [Google Scholar]
- 21.Acar N, Korgun ET, Ustunel I. Cell cycle inhibitor p57 expression in normal and diabetic rat placentas during some stages of pregnancy. Histol Histopathol. 2012;27(1):59–68. doi: 10.14670/HH-27.59. [DOI] [PubMed] [Google Scholar]
- 22.Cho CH, Chen BW, Ho CS, Ko JK, Lam SK. Assessment of hemodynamic changes in rat stomachs by laser Doppler velocimetry and reflectance spectrophotometry. Effects of ethanol and prostaglandin E2 under ischemic and congestive conditions. Digestion. 1994;55(6):389–394. doi: 10.1159/000201170. [DOI] [PubMed] [Google Scholar]
- 23.Konturek PC, Brzozowski T, Burnat G, Szlachcic A, Koziel J, Kwiecien S, Konturek SJ, Harsch IA. Gastric ulcer healing and stress-lesion preventive properties of pioglitazone are attenuated in diabetic rats. J Physiol Pharmacol. 2010;61(4):429–436. [PubMed] [Google Scholar]
- 24.Wallace JL. Prostaglandins, NSAIDs, and gastric mucosal protection: why doesn’t the stomach digest itself? Physiol Rev. 2008;88(4):1547–1565. doi: 10.1152/physrev.00004.2008. [DOI] [PubMed] [Google Scholar]
- 25.Eyries M, Siegfried G, Ciumas M, Montagne K, Agrapart M, Lebrin F, Soubrier F. Hypoxia-induced apelin expression regulates endothelial cell proliferation and regenerative angiogenesis. Circ Res. 2008;103(4):432–440. doi: 10.1161/CIRCRESAHA.108.179333. [DOI] [PubMed] [Google Scholar]
- 26.Han S, Wang G, Qi X, Lee HM, Englander EW, Greeley Jr GH. A possible role for hypoxia-induced apelin expression in enteric cell proliferation. Am J Physiol Regul Integr Comp Physiol. 2008;294(6):R1832–R1839. doi: 10.1152/ajpregu.00083.2008. [DOI] [PubMed] [Google Scholar]
- 27.Das D, Banerjee RK. Effect of stress on the antioxidant enzymes and gastric ulceration. Mol Cell Biochem. 1993;125(2):115–125. doi: 10.1007/BF00936440. [DOI] [PubMed] [Google Scholar]
- 28.Wang T, Leng YF, Zhang Y, Xue X, Kang YQ, Zhang Y. Oxidative stress and hypoxia-induced factor 1alpha expression in gastric ischemia. World J Gastroenterol. 2011;17(14):1915–1922. doi: 10.3748/wjg.v17.i14.1915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Tarnawski AS, Ahluwalia A, Jones MK. Angiogenesis in gastric mucosa: an important component of gastric erosion and ulcer healing and its impairment in aging. J Gastroenterol Hepatol. 2014;29(Suppl 4):112–123. doi: 10.1111/jgh.12734. [DOI] [PubMed] [Google Scholar]
- 30.Akiba Y, Nakamura M, Mori M, Suzuki H, Oda M, Kimura H, Miura S, Tsuchiya M, Ishii H. Inhibition of inducible nitric oxide synthase delays gastric ulcer healing in the rat. J Clin Gastroenterol. 1998;27(Suppl 1):S64–S73. doi: 10.1097/00004836-199800001-00011. [DOI] [PubMed] [Google Scholar]
- 31.Tatemichi M, Ogura T, Sakurazawa N, Nagata H, Sugita M, Esumi H. Roles of inducible nitric oxide synthase in the development and healing of experimentally induced gastric ulcers. Int J Exp Pathol. 2003;84(5):213–220. doi: 10.1111/j.1365-2613.2003.00357.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Langer HF, Chavakis T. Leukocyte-endothelial interactions in inflammation. J Cell Mol Med. 2009;13(7):1211–1220. doi: 10.1111/j.1582-4934.2009.00811.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Kwiecien S, Brzozowski T, Konturek PC, Pawlik MW, Pawlik WW, Kwiecien N, Konturek SJ. Gastroprotection by pentoxyfilline against stress-induced gastric damage. Role of lipid peroxidation, antioxidizing enzymes and proinflammatory cytokines. J Physiol Pharmacol. 2004;55(2):337–355. [PubMed] [Google Scholar]
- 34.Kwiecień S, Pawlik MW, Brzozowski T, Konturek PC, Śliwowski Z, Pawlik WW, Konturek SJ. Nitric oxide (NO)-releasing aspirin and (NO) donors in protection of gastric mucosa against stress. J Physiol Pharmacol. 2008;59(Suppl 2):103–115. [PubMed] [Google Scholar]
- 35.Izgut-Uysal VN, Agac A, Derin N. Effect of carnitine on stress-induced lipid peroxidation in rat gastric mucosa. J Gastroenterol. 2001;36(4):231–236. doi: 10.1007/s005350170108. [DOI] [PubMed] [Google Scholar]
- 36.Izgut-Uysal VN, Bulbul M, Tan R, Derin N, Ustunel I, Agar A, Yargicoglu P. Effect of chronic stress and L-carnitine on rat stomach. J Physiol Sci. 2007;57(3):187–192. doi: 10.2170/physiolsci.RP004707. [DOI] [PubMed] [Google Scholar]
- 37.Foussal C, Lairez O, Calise D, Pathak A, Guilbeau-Frugier C, Valet P, Parini A, Kunduzova O. Activation of catalase by apelin prevents oxidative stress-linked cardiac hypertrophy. FEBS Lett. 2010;584(11):2363–2370. doi: 10.1016/j.febslet.2010.04.025. [DOI] [PubMed] [Google Scholar]
- 38.Than A, Zhang X, Leow MK, Poh CL, Chong SK, Chen P. Apelin attenuates oxidative stress in human adipocytes. J Biol Chem. 2014;289(6):3763–3774. doi: 10.1074/jbc.M113.526210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Pisarenko O, Shulzhenko V, Studneva I, Pelogeykina Y, Timoshin A, Anesia R, Valet P, Parini A, Kunduzova O. Structural apelin analogues: mitochondrial ROS inhibition and cardiometabolic protection in myocardial ischaemia reperfusion injury. Br J Pharmacol. 2015;172(12):2933–2945. doi: 10.1111/bph.13038. [DOI] [PMC free article] [PubMed] [Google Scholar]


