Abstract
The present study was to investigate whether there are functional connections between the hypothalamic supraoptic nucleus (SON) and the stomach, which is the case with the paraventricular nucleus. The rats were divided into four groups. Group I: the neuronal discharge was recorded extracellularly in the NTS, DMV or SON before and after cold physiological saline (4°C) was perfused into the stomach and effused from the duodenum. Group II: the rats were stimulated as for Group I and c-Fos expression in NTS, DMV and SON was examined. Group III: the control to Group II. Group IV: gastric motility was recorded continuously before and after microinjection of l-Glu into the SON. In Group I, the discharge frequency increased in all the three nuclei, while in Group II, Fos expression in NTS, DMV and SON was, respectively, greater than that of Group III. In Group IV, microinjection of l-Glu (5 nmol) into SON significantly inhibited gastric motility. These data suggest there are functional connections between SON and stomach.
Keywords: Supraoptic nucleus, Neuronal discharge, c-Fos expression, Gastric motility
Introduction
Hypothalamus is the center for neuroendocrine which combines neuromodulation and humoral regulation to modulate the visceral functions, such as body temperature, feeding, reproduction, osmotic pressure, endocrine and so on. From the rostral to the caudal end, it is composed of the preoptic, supraoptic, tuberal and bulla fornicis regions. The supraoptic nucleus (SON) and the paraventricular nucleus (PVN) are the most prominent nuclei in the anterior hypothalamus, and their neuronal morphology and physiology are similar in Golgi-stained or immunohistochemistry studies [1], both being mainly composed of vasopressinergic (VP) and oxytocinergic (OT) neurons. It is well known that the VP and OT will be released and will combine with the target organ when, for example, the osmotic pressure of plasma increases, lactates or delivers [2, 3].
However, more and more evidence confirms that there are functional connections between the PVN and the stomach. Rogers et al. found that stimulation of the PVN reduced gastric acid secretion [4] and inhibited gastric motility [5], and the latter standpoint was supported by Sakaguchi [6]. Humphreys et al. [7] reported that injection of neuropeptide Y into the PVN inhibited gastric acid secretion in the rat, and gastric motility and food intake was also elevated after lesions of the PVN in the rat [8]. The effects of the PVN on the stomach can be abolished by bilateral subdiaphragmatic vagotomy, so we can conclude that the PVN modulates the stomach via vagal parasympathetic nerves which come from the neurons located in the dorsal motor nuclei of the vagus (DMV) and partly in the nucleus ambiguus (NA). Sofroniew et al. [9–11] confirmed there were direct projections from OT and VP neurons in the PVN to the DMV via immunohistochemical visualization of the horseradish peroxidase and neuroregulator. So far, it remains unknown whether there are functional connections between the SON and the stomach.
Our prior studies investigated the c-Fos expression in medulla oblongata and hypothalamus nuclei during different durations (30, 60, 120, 180 min) of restraint water-immersion stress (RWIS) in cold water (21 ± 1°C) in the rat. The most intense c-Fos induction was always observed in the SON and the PVN in the hypothalamus, as well as the DMV, NA and NTS in the medulla oblongata [12, 13]. These data strongly suggest that the SON, PVN, DMV, NA, NTS participate in the disorders of gastric motility and secretion induced by the RWIS, and much evidence indicates that there are functional connections between all of the nuclei mentioned above and the stomach except for the SON [8, 14–19].
Mersereau [20] found that it was hypothermia that resulted in the disorders of gastric motility and secretion in RWIS, and our prior work confirmed this. However, the thermoreceptors (e.g., dermal or viscera thermoreceptors) were excited when the rats were restrained and immersed in the cold water. So what happens in the DMV, NTS and especially in the SON when the gastric thermoreceptors are activated by cold water only? And vice versa, what happens to gastric motility when the SON is activated? In other words, it remains unknown whether there are bidirectional functional connections between the SON and the stomach.
The purpose of our study was therefore to lower the gastric temperature by perfusing cold water (4°C physiological saline, normally used to preduce irritable bowel syndrome [21]) into the stomach, and then take the neuronal discharge and c-Fos expression as parameters to investigate the excitability of the DMV, NTS and SON. In addition, we microinjected l-Glu, a neuron body incitant that can be visualized in the SON, into the SON to investigate the effects on gastric motility. Thus, we set out to obtain evidence of functional connections between the SON and the stomach, so deriving more data about the mechanism of gastric modulation and function of the hypothalamus SON from our experiment.
Materials and methods
Experiments animals
Male adult Wistar rats (Experiment Animal Center of Shandong University, Jinan, China), weighing 270–320 g, were individually housed in cages at an environmental temperature of 22 ± 2°C and normal day/night cycle. They were allowed to have pelleted food and tap water ad libitum. Before the experiments, they were fasted for 24 h, but allowed free access to water. A thermostatically controlled heating lamp was used to maintain rectal temperature between 37 and 39°C during the experiments. All procedures were performed in accordance with the Japanese Physiological Society’s Guiding Principles for the Care and Use of Animals in the Field of Physiological Sciences.
Then, the rats were divided randomly into four groups. Group I: neuronal discharge was recorded; Group II: c-Fos expression was examined after stimulus; Group III: the control to group II; Group IV: gastric motility was recorded while microinjecting l-Glu into the SON.
Surgical preparation
The rats were anesthetized with 20% urethane dissolved in 0.9% NaCl solution (1.0 g/kg) via intraperitoneal injection. If necessary, 0.2 ml supplements were administered to maintain the animal unresponsive to toe pinch. The rats were placed supine and the trachea cannula intubated to maintain a patent airway and to provide artificial respiration, if necessary, to avoid stifling to death. Then, the esophagus was cannulated with a polyethylene tube for the perfusion of cold physiological saline (4°C), and another polyethylene tube (diameter 3 mm) was inserted into the stomach through a small incision in the duodenum for the outflow.
Electrophysiological recordings
The surgically prepared rat (Group I) was placed in a Stoelting 51600 stereotaxic apparatus (Stoelting, USA) with the incisor bar 3.3 mm below the center of the ear bars. The dorsal surface of the brain was exposed by limited occipital (for the localization of the DMV and NTS) or parietal (for the localization of the SON) craniotomy. The cerebral dura mater and arachnoid were carefully removed and then covered with agar (3–4% in saline) to improve stability for neuronal discharge recording [22]. The coordinates of the NTS, DMV or SON were as defined by the coronal diagram [23] (e.g. for the SON, the stereotaxic coordinates were at a level 1.1 mm posterior to the bregma, 1.8 mm right lateral to the midline and a depth of 9.3 mm below the surface of the skull). However, as we know, the coordinates may not be entirely accurate, so a little modification was usually needed according to experimental experience of histologically identifying the stimulated site. The recording glass microelectrode (tip diameter 1 μm, resistance 8–20 MΩ) filled with 6.8% sodium acetate and 2% Pontamine sky blue was inserted vertically into the right side of the brain. Then, the recording microelectrode was connected to the microelectrode bridge amplifier via a piece of silver wire (diameter 0.6 mm), and in turn to the BL-420F Biological Experimental System (Chengdu Taimeng, China). When the neuronal discharge was steady, cold physiological saline (4°C) was perfused into the stomach through the esophagus and effused from the duodenum for 5 min at 10 mL/min, while the current was mellifluent so as to avoid gastric distension. The discharge frequency diversity was displayed on the computer. To identify the site of the recording electrode, a negative current (10 μA, 10 min) was passed through the electrode to form an iron deposit of Pontamine sky blue at the end of each experiment. Then, 0.9% sodium chloride solution and 10% formalin solution were perfused into the aorta and outflowed from the right atrium. The brain was then removed and fixed in 10% formalin for 2–3 days. Frozen serial coronal sections (40 μm) were cut and stained with Neutral Red and examined microscopically. Only the results for which the tips of the recording electrode were just within the nucleus of the DMV, NTS or SON were used for statistical analysis.
c-Fos expression
Ten rats were randomly divided into two groups designated according to stimulus or not. In Group II, the surgically prepared rats were stimulated with cold physiological saline, the method being the same as the electrophysiological recording group (Group I), and then placed on a table for 60 min so as to express the c-Fos intensively. Group III rats (the control group for Group II) were processed in the same way as Group II except for the cold water stimulus.
Afterwards, 0.01 mol/L phosphate buffered saline (PBS, pH 7.4) followed by 500 ml freshly prepared 4% paraformaldehyde in 0.1 mol/L phosphate buffer (4°C) were perfused into the ascending aorta and outflowed from the right atrium. The brain was then removed and fixed in 4% paraformaldehyde for 24 h, and immersed in 0.1 mol/L PB containing 20% sucrose for 48 h. The brain was cut into 35-μm coronal sections serially in a cryostat and collected in 0.1 mol/L PBS containing 0.3% Triton X-100 (PBST). The free-floating sections were pretreated for 30 min in methanolic 3% H2O2 to eliminate endogenous peroxidase activity. After rinsing three times with 0.01 mol/L PBS, they were incubated with blocking buffer (5% normal goat serum and 0.3% Triton X-100 in PBS) for 30 min, and with rabbit anti-c-Fos antibody (sc-52; Santa Cruz Biotechnology, Santa Cruz, CA, USA) at a dilution of 1:2,000 for 24 h at 4°C. They were incubated with biotinylated goat anti-rabbit IgG (Zymed Laboratories, San Fransisco, CA, USA) for 1.5 h at room temperature and then with streptavidin–biotin–horseradish peroxidase complex (Zymed) for 1.5 h at room temperature. The sections were submitted to a diaminobenzidine reaction, yielding a brown nuclear deposit. Between steps, the sections were rinsed completely in PBS containing 1% Triton X-100 (PBST). Sections were mounted on gelatin-coated glass slides, counterstained with hematoxylin, dehydrated in a series of graded alcohols, cleared in xylene, and coverslipped [9].
Effects of microinjection of l-Glu into SON on gastric motility
The rats of group IV were anesthetized and a midline laparotomy was performed, a latex balloon made of condom attached to polyethylene tube being inserted into the pylorus through the small incision in the forestomach wall. The balloon was inflated with warm physiological saline (0.2–0.5 ml) while the polyethylene tube was connected to a pressure transducer, which, in turn, was connected to data acquisition system (Chengdu Taimeng). The gastric motility curves were recorded and displayed continuously on the computer.
The rat was placed in stereotaxic apparatus as for the electrophysiological recordings. A glass micropipette (the tip diameter 30–50 μm) filled with l-Glu 5 nmol in 0.05 μl was inserted vertically into the right SON. The gastric motility was recorded for 30 min before injection, and then l-Glu was microinjected into the SON within 1 min and followed by recording the gastric motility for 30 min. At the end of the experiments, the site of the microinjection was examined in the same way as for the electrophysiological recordings.
Data analysis
In Group I, we compared the mean discharge frequency (spikes/s) during the course of 3 min after stimulus with that before stimulus. The difference between the two groups was evaluated by paired t test. In Groups II and III, the total numbers of c-Fos positive neurons were counted unilaterally in each brain nucleus and a mean value of each brain nucleus was determined by samples from ten randomly selected sections cut through the specific brain nuclei, which were identified by the Paxinos and Watson’s atlas coordinates [23]. The difference between the experimental and control groups was evaluated by t test. In Group IV, the total amplitude, total duration, and motility index of gastric contraction waves within 10 min before and after microinjection were measured and evaluated by paired t test. The motility index (mmHg s) was defined as the product of amplitude and duration of every contraction wave, while the inhibitory rate = (the value before stimulation − the value after microinjection)/the value before stimulation. All data were presented as mean ± SD and analyzed with SPSS13.0 software (SPSS, Chicago, IL, USA). P < 0.05 was considered statistically significant, and P < 0.01 highly significant.
Results
Location of the neuronal recording sites
The brain sections were stained with neutral red and then the DMV, NTS and SON were confirmed by histological analysis according to Paxinos and Watson’s atlas coordinates (Fig. 1).
Fig. 1.
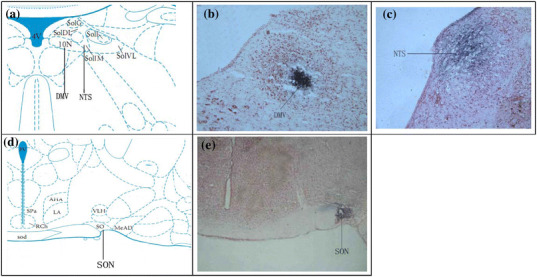
Photomicrograph of the DMV, NTS and SON recording site (indicated by arrows). a Representative site of DMV and NTS in the brain atlas. b Blue macula indicates DMV. c Blue macula indicates NTS. d Representative site of SON. e Blue macula indicates SON
Effects of 4°C physiological saline stimulus on discharge frequencies of the DMV, NTS and SON
The discharge frequencies caused by the lower temperature of all three nuclei increased, this phenomenon arose in about 10 s and lasted 5–10 min, but differed in degree. The diagrams of increasing frequency were similar to each other (Fig. 2). Compared with before stimulus, the discharge frequencies of DMV increased from 2.46 ± 1.05 to 3.54 ± 0.87, or 43.90% (n = 5, P < 0.05), while for the SON (n = 7) there was an increase from 4.50 ± 0.68 to 6.85 ± 1.71, or 52.2% (P < 0.05). The discharge frequencies of NTS increased from 2.27 ± 1.20 to 3.00 ± 1.15 (n = 5), or 32.16%, which showed no significant difference (Fig. 3).
Fig. 2.
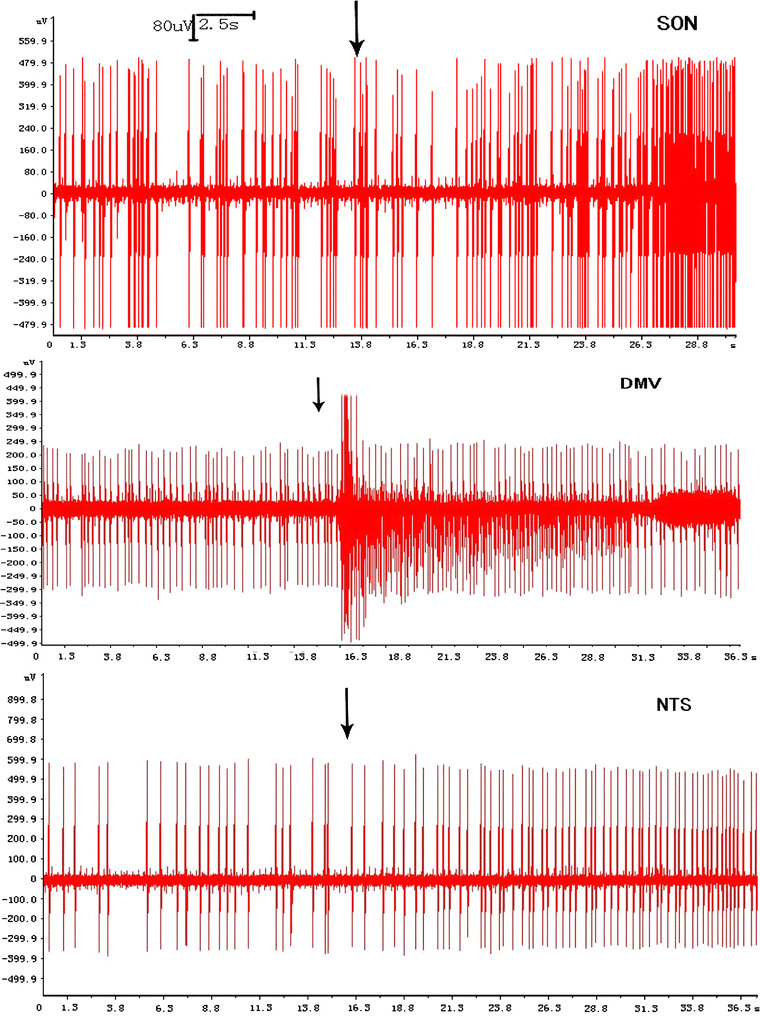
Representative discharge diagram of the SON (n = 7), DMV (n = 5) and NTS (n = 5). Compared with before microinjection, the discharge frequencies of all three nuclei increased, the black arrow represents the cold saline stimulus, the x-axis represents time (s), and the y-axis represents amplitude of spikes (μV)
Fig. 3.
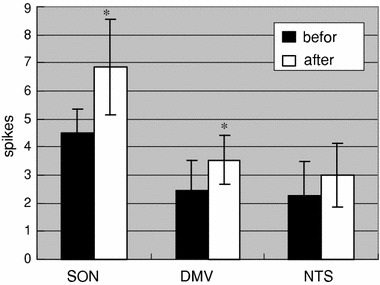
Effects of 4°C physiological saline stimulus on discharge frequency of the SON (n = 7), DMV (n = 5) and NTS (n = 5), 180 s after stimulus versus before stimulus; y-axis represents discharge frequency (spikes/s), *P < 0.05
c-Fos expression in DMV, NTS and SON
To examine the effects of brain nuclei responsible for 4°C physiological saline stimulus in the stomach, we studied c-Fos expression in the SON, DMV and NTS using immunohistochemical staining (Fig. 4).We observed that c-Fos positive cells in Group II were more numerous than in Group III being highly significantly different in the SON and DMV and significantly different in the NTS (Table 1). Moreover, c-Fos expression of the SON was the most intense among the three nuclei.
Fig. 4.
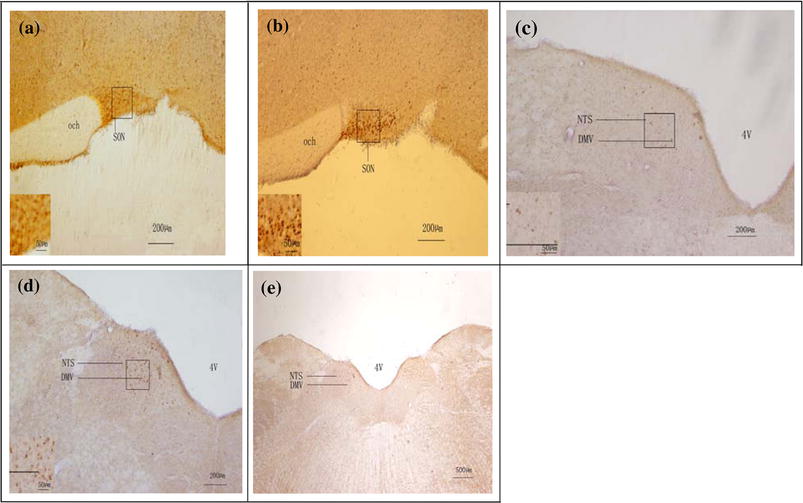
c-Fos expression of experimental group (Group II, n = 5) and control group (Group III, n = 5) in the SON, NTS, and DMV (indicated by arrows). The insets show higher magnification of cells in the small boxes. a c-Fos expression of control group in the SON. b c-Fos expression of experiment group in the SON. c c-Fos expression of control group in the NTS and DMV. d c-Fos expression of experiment group in the NTS and DMV. e Full view picture of the NTS and DMV (4V fourth ventricle)
Table 1.
The numbers of c-Fos positive cells in brain nuclei
| SON | DMV | NTS | |
|---|---|---|---|
| Group III (n = 5) | 102.82 ± 7.39* | 22.01 ± 2.93 | 127.8 ± 9.74 |
| Group II (n = 5) | 188.23 ± 16.45* | 53.22 ± 6.93** | 169.03 ± 16.55* |
| Increasing rate | 82.88% | 141.81% | 32.23% |
Values are mean ± SD, the numbers of c-Fos positive neurons were counted unilaterally in each brain nucleus, a mean value of each brain nucleus was determined by samples from ten randomly selected sections cut through the specific brain nuclei
* P < 0.05, ** P < 0.01 compared with the control group
Effects of entogastric cold saline perfusion on rectum and gastric temperature
Rectum and gastric temperature were recorded continuously before and after the stimulus, and we observed that the gastric temperature decreased abruptly (P < 0.01), but the rectum temperature was only slightly lower (Table 2).
Table 2.
Effects of stimulus on rectum and gastric temperature
| Before stimulus (°C) | After stimulus (°C) | |
|---|---|---|
| Stomach (n = 22) | 35.5 ± 1.5 | 25.0 ± 1** |
| Rectum (n = 22) | 36.5 ± 0.5 | 35.5 ± 0.5 |
The gastric temperature decreased abruptly, but the rectum temperature was only lower slightly
** P < 0.01
Effects of l-Glu microinjected into the SON on gastric motility
Microinjection of l-Glu (5 nmol, n = 7) into the SON gradually but significantly inhibited gastric motility (Fig. 5). The inhibition lasted about 20–30 min. The amplitude of each wave was 8–40 mmHg, and the total amplitude decreased from 103.1 ± 42.6 to 88.1 ± 41.3 mmHg, the inhibitory rate being 14.5% (P < 0.05). The motility index (mmHg s) decreased from 1,482.1 ± 708.2 to 1,185.5 ± 639.2, the inhibitory rate being 13.53–42.52% in different rats (P < 0.05). The gastric motility frequency and total duration decreased slightly, and the duration of each wave was 12–15 s over all experiments (Table 3).
Fig. 5.
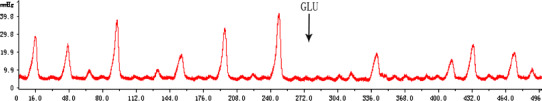
Inhibitory response of gastric motility to unilateral microinjection of l-glutamate (5 nmol, 0.05 μl) into the SON in the anesthetized rats (n = 7); the arrow represents microinjection, x-axis represents time (s), y-axis represents amplitude of gastric motility (mmHg)
Table 3.
Effects of l-Glu microinjected into SON on gastric motility (n = 7)
| Before stimulus | After stimulus | Inhibitory rate (%) | |
|---|---|---|---|
| Frequency | 9.2 ± 3.8 | 7.7 ± 2.7 | 16.3 |
| Total amplitude (mmHg) | 103.1 ± 42.6 | 88.1 ± 41.3* | 14.5 |
| Total duration (s) | 137.9 ± 61.7 | 120.1 ± 48.1 | 12.4 |
| Motility index | 1482.1 ± 708.2 | 1185.5 ± 639.2* | 18.4 ± 15.5 |
Values presented are mean ± SD. The gastric motility frequency and total duration decreased slightly, but the total amplitude and motility index decreased significantly
* P < 0.05
Discussion
The discharge frequencies of all three nuclei caused by the cold saline stimulation of the stomach increased, which suggested that they were activated when the gastric wall thermoreceptors were excited. c-Fos protein, a nuclear phosphoprotein product of the immediate early gene c-fos, has been extensively used as a marker of neuronal activation, and will express most intensely in 60 min. So the results of c-Fos expression were consistent with the implications of increasing discharge frequencies. When l-Glu, a neuron body incitant in brain, was microinjectedinto the SON, the gastric motility was inhibited. As for what will happen when the SON are inhibited, e.g., by microinjecting GABA, needs to be further confirmed in a future study.
Effects of cold saline perfusing the stomach on discharge and c-Fos expression of the NTS and DMV
The increase of discharge and c-Fos expression under the stimulus (gastric temperature decreased instead of rectal temperature) indicated that the gastric wall thermoreceptors were excited and, in turn, activated the DMV and NTS. It is well known that the stomach wall is richly innervated by mechanosensory and chemosensory nerve terminals [24]. When the stomach was stimulated by mechanical distention or by chemical composition in food, this information ascends to the NTS in the medulla via the vagus nerve, then the NTS projects to theDMV, and also to the PVN and partly to the limbic lobe of the anterior hypothalamus and pallium. The present study showed that the impulse of thegastric wall thermoreceptors ascended to the NTS via the vagus nerve, then subsequently to the DMV and SON, which has been mentioned in few other reports.
Effects of cold saline perfusing the stomach on the neuronal activity of the SON
It is well known that there are fiber projections from the DMV to the stomach and from the stomach to the NTS directly via the vagus nerve, but there are no nerve fibers between the stomach and the SON. However, both the discharge frequency (increasing rate 52.2%, P < 0.05) and c-Fos expression (compared with the control group, the increasing rate was 82.88%, P < 0.01) showed that the SON neurons were activated, implying that there were functional connections between the SON and the stomach. This standpoint was supported by other research: we microinjected horseradish peroxidase into the NTS for retrograde tracing the nerve tract to the hypothalamus, and we found labeled neurons in the PVN and SON of the rat (unpublished data). On these grounds, we conclude that the impulse from the NTS directly activated the SON when the stomach was perfused with cold saline.
The neuronal mechanism of the SON inhibited gastric motility
One of the major findings of the present study is that microinjection of l-Glu into the right SON inhibited gastric motility, which supported the conclusion that there are functional connections between the stomach and the SON. In other studies, gastric secretion increased after direct microinjecting of methacholine chloride, a kind of incentive chemical stimulation, into the SON in dogs, and this phenomenon could be eliminated after bilateral vagotomy [25]. Ueta et al. [26] consistently observed that discharge of VP neurons were inhibited by gastric distension induced by balloon inflation of 4–8 ml in the rat. Takayuki discovered that the gastric distention of physiological levels stimulated the vascular release of 5-HT, and induced c-Fos expression (representing neuronal activity) in the NTS, the area postrema (AP), the PVN and the SON, and could be blocked by truncal vagotomy, from which he assumed that there were fiber projections between the NTS and the SON [27]. These results are consistent with the present study that the SON has functional connections with the stomach.
Rogers and Hermann [5] microinjected OT into the DMV of rats and observed a reduction in gastric motility that was abolished by bilateral vagotomy (our research group microinjected VP into the DMV and got the same result; unpublished data). OT and VP receptors could be visualized via immunohistochemistry in the DMV. These results were consistent with the present study. Therefore, we assume that SON neurons project to the DMV and form synapses with the cholinergic preganglionic neurons in the DMV which innervate the stomach smooth muscle. However, Mohammed et al. [28] reported microinjection of OT into veins directly reduced the gastric secretion and gastric motility was inhibited even though the vagus nerve was cut off beforehand [29].
In summary, these data strongly suggest that there are functional connections between the SON and the stomach in rats. Much evidence indicates that there are similarities between the SON and the PVN in the localization, neuron organization, fiber projections, neuron generation cycle and biochemical specificity [30–35]. However, whether the SON modulates gastric motility via activating the DMV needs to be further confirmed in future studies.
Acknowledgments
This work was supported by the National Science Foundation of China (No. 30770277, 30970354, 31071920).
References
- 1.Armstrong WE, Smith BN, Tian M. Electrophysiological characteristics of immunochemically identified rat oxytocin and vasopressin neurons in vitro. J Physiol. 1994;475:115–128. doi: 10.1113/jphysiol.1994.sp020053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Stern JE, Armstrong WE. Changes in the electrical properties of supraoptic nucleus oxytocin and vasopressin neurons during lactation. J Neurosci. 1996;16:4861–4871. doi: 10.1523/JNEUROSCI.16-16-04861.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Fuchs AR, Saito S. Pituitary oxytocin and vasopressin content of pregnant rats before, during and after parturition. Endocrinology. 1971;88:574–578. doi: 10.1210/endo-88-3-574. [DOI] [PubMed] [Google Scholar]
- 4.Rogers RC, Hermann GE. Hypothalamic paraventricular nucleus stimulation-reduced gastric acid secretion and bradycardia suppressed by oxytocin antagonist. Peptides. 1986;7:695–700. doi: 10.1016/0196-9781(86)90046-X. [DOI] [PubMed] [Google Scholar]
- 5.Rogers RC, Hermann GE. Oxytocin, oxytocin antagonist, TRH, and hypothalamic paraventricular nucleus stimulation effects on gastric motility. Peptides. 1987;8:505–513. doi: 10.1016/0196-9781(87)90017-9. [DOI] [PubMed] [Google Scholar]
- 6.Sakaguchi T, Ohtake M. Inhibition of gastric motility induced by activation of the hypothalamic paraventricular nucleus. Brain Res. 1985;335:365–367. doi: 10.1016/0006-8993(85)90495-0. [DOI] [PubMed] [Google Scholar]
- 7.Humphreys GA, Davison JS, Veale WL. Injection of neuropeptide Y into the paraventricular nucleus of the hypothalamus inhibits gastric acid secretion in the rat. Brain Res. 1988;456:241–248. doi: 10.1016/0006-8993(88)90223-5. [DOI] [PubMed] [Google Scholar]
- 8.Flanagan LM, Dohanics J, Verbalis JG, Stricker EM. Gastric motility and food intake in rats after lesions of hypothalamic paraventricular nucleus. Am J Physiol. 1992;263:R39–R44. doi: 10.1152/ajpregu.1992.263.1.R39. [DOI] [PubMed] [Google Scholar]
- 9.Sofroniew MV, Schrell U. Evidence for a direct projection from oxytocin and vasopressin neurons in hypothalamic paraventricular nucleus to the medulla oblongata immunohistochemical visualization of both the horseradish peroxidase transported and the peptide produced by the same neurons. Neurosci Lett. 1981;22:211–217. doi: 10.1016/0304-3940(81)90108-7. [DOI] [Google Scholar]
- 10.Swanson LW, Sawchenko PE. Hypothalamic integration organization of the paraventricular and supraoptic nucleus. Annu Rev Neurosci. 1983;6:269–324. doi: 10.1146/annurev.ne.06.030183.001413. [DOI] [PubMed] [Google Scholar]
- 11.Zhang JF, Zhang YM, Yan CD, Zhou ZP. Neuroregulative mechanism of hypothalamic paraventricular nucleus on gastric ischemia–reperfusion injury in rats. Life Sci. 2002;71:1501–1510. doi: 10.1016/S0024-3205(02)01850-7. [DOI] [PubMed] [Google Scholar]
- 12.Zhang YY, Cao GH, Zhu WX, Cui XY, Ai HB. Comparative study of c-Fos expression in rat dorsal vagal complex and nucleus ambiguus induced by different durations of restraint water-immersion stress. Chinese J Physiol. 2009;52:143–150. doi: 10.4077/cjp.2009.amh045. [DOI] [PubMed] [Google Scholar]
- 13.Zhang YY, Cao GH, Zhu WX, Cui XY, Ai HB. c-Fos expression in the supraoptic nucleus is the most intense during different durations of restraint water-immersion stress in the rat. J Physiol Sci. 2009;59:367–375. doi: 10.1007/s12576-009-0044-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Alberto R, Hermann E, Browning N, Rogers C. Brainstem circuits regulating gastric function. Annu Rev Physiol. 2006;68:279–305. doi: 10.1146/annurev.physiol.68.040504.094635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Zhou SY, Lu YX, Yao HR, Owyang C. Spatial organization of neurons in the dorsal motor nucleus of the vagus synapsing with intragastric cholinergic and nitric oxide/VIP neurons in the rat. Am J Physiol Gastrointest Liver Physiol. 2008;294:G1201–G1209. doi: 10.1152/ajpgi.00309.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Cruz T, Murphy C, Niaz S, Verbalis G, Gillis A. A reevaluation of the effects of stimulation of the dorsal motor nucleus of the vagus on gastric motility in the rat. Am J Physiol Regul Integr Comp Physiol. 2007;292:291–307. doi: 10.1152/ajpregu.00863.2005. [DOI] [PubMed] [Google Scholar]
- 17.Wang YH, Ai HB, Zhang YY, Cui XY. Effects and mediated pathway of electrical stimulation of nucleus ambiguus on gastric motility and mucus secretion in rats. Scand J Clin Lab Invest. 2007;67:489–497. doi: 10.1080/00365510601161505. [DOI] [PubMed] [Google Scholar]
- 18.Sun HZ, Zhao SZ, Cui XY, Ai HB. Effects and mechanisms of l-glutamate microinjected into nucleus ambiguus on gastric motility in rats. Chin Med J. 2010;123:1052–1057. [PubMed] [Google Scholar]
- 19.Zhang XY, Ai HB, Cui XY. Effects of nuclei ambiguus and dorsal motor nuclei of vagus on gastric H+ and HCO3 secretion in rats. World J Gastroenterol. 2006;12:3271–3274. doi: 10.3748/wjg.v12.i20.3271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Mersereau WA. Hypothermia-induced gastric hypercontractility in the genesis of the restraint ulcer. Can J Surg. 1981;24:622–625. [PubMed] [Google Scholar]
- 21.Funakami Y, Itoh E, Wada T, Ichida S. Specific alternation of rhythm in temperature (SART) stress-induced irritable bowel syndrome-like changes in mice and effects of drugs. Biol Pharm Bull. 2010;33:1545–1549. doi: 10.1248/bpb.33.1545. [DOI] [PubMed] [Google Scholar]
- 22.Wang WG, Chen X, Jiang H, Jiang ZY. Effects of ghrelin on glucose-sensing and gastric distension sensitive neurons in rat dorsal vagal complex. Regul Peptides. 2008;146:169–175. doi: 10.1016/j.regpep.2007.09.007. [DOI] [PubMed] [Google Scholar]
- 23.Paxinos G, Watson C. The rat brain in stereotaxic coordinates. 4. Sydney: Academic; 1998. [DOI] [PubMed] [Google Scholar]
- 24.Bear MF. Neuroscience: exploring the brain. 2. Beijing: Higher Education Press; 2002. [Google Scholar]
- 25.Zawoiski EJ, Koplovitz I. Gastric secretory response of the anesthetized dog after direct chemical stimulation of the supraoptic region. Exp Neurol. 1977;55:122–132. doi: 10.1016/0014-4886(77)90164-9. [DOI] [PubMed] [Google Scholar]
- 26.Ueta Y, Kannan H, Yamashita H. Gastric afferents to paraventricular nucleus in the rat. Exp Brain Res. 1991;84:487–494. doi: 10.1007/BF00230960. [DOI] [PubMed] [Google Scholar]
- 27.Mazda T, Yamamoto H, Fujimura M, Fujimiya M. Gastric distension-induced release of 5-HT stimulates c-fos expression in specific brain nuclei via 5-HT receptors in conscious rats. Am J Physiol Gastrointest Liver Physiol. 2004;287:G228–G235. doi: 10.1152/ajpgi.00373.2003. [DOI] [PubMed] [Google Scholar]
- 28.Mohammed A, Shewade G, Koumaravelou K, Abraham K, Sadashivam V, Subramaniam R. Gastric antisecretory and antiulcer activity of oxytocin in rats and guinea pigs. Life Sci. 2001;70:17–24. doi: 10.1016/S0024-3205(01)01376-5. [DOI] [PubMed] [Google Scholar]
- 29.Hashmonai M, Torem S, Argov S, Barzilai A, Schramek A. Prolonged post-vagotomy gastric atony treated by oxytocin. Br J Surg. 1979;66:550–551. doi: 10.1002/bjs.1800660809. [DOI] [PubMed] [Google Scholar]
- 30.Sawchenko PE, Swanson LW. The organization of forebrain afferents to the paraventricular and supraoptic nuclei of the rat. J Comp Neurol. 2004;218:121–144. doi: 10.1002/cne.902180202. [DOI] [PubMed] [Google Scholar]
- 31.Mark L, glenn I. Collateral Input to the paraventricular and supraoptic nuclei in rat. I. Afferents from the subfornical organ and the anteroventral third ventricle region. Brain Res Bull. 1990;24:231–238. doi: 10.1016/0361-9230(90)90210-Q. [DOI] [PubMed] [Google Scholar]
- 32.Zlmmerman EA, Ndaver G, Hou-Yu A, Silverman AJ. Vasopressinergic and oxytocinergic pathways in the central nervous system. Fed Proc. 1984;43:91–96. [PubMed] [Google Scholar]
- 33.Swaab DF, Nijveldt F, Pool CW. Distribution of oxytocin and vasopressin in the rat supraoptic and paraventricular nucleus. J Endocrinol. 1975;67:461–462. doi: 10.1677/joe.0.0670461. [DOI] [PubMed] [Google Scholar]
- 34.Hrabovszky E, Kalló I, Hajszán T, Shughrue PJ, Merchenthaler I, Liposits Z. Expression of estrogen receptor-β messenger ribonucleic acid in oxytocin and vasopressin neurons of the rat supraoptic and paraventricular nuclei. Endocrinol. 1998;139:2600–2604. doi: 10.1210/endo.139.5.6024. [DOI] [PubMed] [Google Scholar]
- 35.Fliers E, Swaaba DF, Poola CW, Verwera RW. The vasopressin and oxytocin neurons in the human supraoptic and paraventricular nucleus; changes with aging and in senile dementia. Brain Res. 1985;342:45–53. doi: 10.1016/0006-8993(85)91351-4. [DOI] [PubMed] [Google Scholar]


