Abstract
Astrocytes have multiple functions such as provision of nourishment and mechanical support to the nervous system, helping to clear extracellular metabolites of neurons and modulating synaptic transmission by releasing gliotransmitters. In excitable cells, voltage-gated K+ (Kv) channels serve to repolarize during action potentials. Astrocytes are considered non-excitable cells since they are not able to generate action potentials. There is an abundant expression of various Kv channels in astrocytes but the functions of these Kv channels remain unclear. We examined whether these astrocyte Kv channels regulate astrocyte “excitability” in the form of cytosolic Ca2+ signaling. Electrophysiological examination revealed that neonatal rat cortical astrocytes possessed both delayed rectifier type and A-type Kv channels. Pharmacological blockade of both delayed rectifier Kv channels by TEA and A-type Kv channels by quinidine significantly suppressed store-operated Ca2+ influx; however, TEA alone or quinidine alone did not suffice to cause such suppression. TEA and quinidine together dramatically enhanced current injection-triggered membrane potential overshoot (depolarization); either drug alone caused much smaller enhancements. Taken together, the results suggest both delayed rectifier and A-type Kv channels regulate astrocyte Ca2+ signaling via controlling membrane potential.
Keywords: Astrocyte, Ca2+ signaling, Membrane potential, Voltage-gated K+ channels
Introduction
Besides the roles of nourishing and providing mechanical support for the neurons, astrocytes also serve to clear neurotransmitters and maintain extracellular K+ homeostasis [1, 2]. Astrocytes are classified as non-excitable cells, since they are not able to generate action potentials. Nevertheless, astrocytes display “excitation” in the form of cytosolic Ca2+ signaling attributable to the expression of neurotransmitter receptors, some of which are ionotropic (Ca2+-permeable) or metabotropic (generation of inositol-1,4,5-trisphosphate) receptors [3]. Astrocytes, stimulated by neurotransmitters, may release gliotransmitters such as glutamate, d-serine and ATP, which could in turn modulate synaptic transmission [4, 5]. Therefore, astrocytes are not merely passively supportive cells but are cells taking active roles in modulating neural processes.
In excitable cells, voltage-gated K+ (Kv) channels are responsible for K+ efflux, which accounts for repolarization in an action potential. Intriguingly, astrocytes possess abundant Kv channels [2]; the latter’s functions in astrocytes are unclear. In spinal cord astrocytes, current injection triggers an “action potential-mimicking voltage overshoot” [6]. Subsequent repolarization is inhibited by a Kv channel blocker, 4-aminopyridine (4-AP), suggesting that Kv channels may play a role in regulating membrane potential. Since changes in membrane potential are expected to affect Ca2+ entry (depolarization and hyperpolarization decreases and increases, respectively, the electrical driving force for Ca2+ influx in non-excitable cells), it is possible that Kv channel activities could regulate Ca2+ influx in astrocytes. This possibility has remained unexplored. In this report, we investigated whether Kv channel activities regulated Ca2+ signaling in rat cortical astrocytes.
Methods
Chemicals
Dulbecco’s modified Eagle’s medium (DMEM), fetal calf serum, and tissue culture reagents were purchased from Invitrogen Corporation (Carlsbad, CA, USA). Roswell Park Memorial Institute (RPMI) 1640 medium was from Gibco. Tetraethylammonium chloride (TEA), quinidine and cyclopiazonic acid (CPA) were from Sigma-Aldrich (St. Louis, MO). Fura-2 AM was from EMD Millipore (Billerica, MA).
Cell culture
Primary rat astroctye culture was prepared according to a previous report [7] with a slight modification. Briefly, mixed-glia cultures were first prepared from brains of 1-day-old pups of Sprague–Dawley rats. Mechanically dissociated brain cells (5–7 × 107) were seeded into 75-cm2 culture flasks in DMEM/F12 containing 10 % heat-inactivated fetal bovine serum (FBS), 2 mM l-glutamine, 1 mM sodium pyruvate, 100 μM non-essential amino acids, 100 U/ml penicillin and 100 μg/ml streptomycin. Cell cultures were maintained at 37 °C in a humidified atmosphere of 5 % CO2 and 95 % air, and medium was replenished twice a week. Once confluence had occurred (usually 12–14 days later), microglia were detached from astrocytes by shaking the flasks at a speed of 180 rpm for 5 h. The cultures were then treated with l-leucine methyl ester, which removed microglia from astrocytes. Experiments were performed by using confluent astrocytes grown in the second passage. Afterward, astrocytes were then detached with trypsin and seeded in DMEM containing 10 % FBS, 100 U/ml penicillin and 100 μg/ml streptomycin. The astrocyte cultures were confirmed by immunocytochemical staining against glial fibrillary acidic protein, resulting in >97 % purity.
Lung epithelial H1355 cells were cultured at 37 °C in 5 % CO2 in RPMI 1640 medium with 10 % FBS and penicillin–streptomycin (100 U/ml, 100 µg/ml) (Invitrogen).
Microfluorimetric measurement of cytosolic Ca2+
Microfluorimetric measurement of cytosolic Ca2+ concentration was performed using fura-2 as the Ca2+-sensitive fluorescent dye as described previously [8]. Briefly, cells were incubated with 5 μM fura-2 AM (Invitrogen, Carlsbad, CA) for 1 h at 37 °C and then washed and bathed in extracellular bath solution which contained (mM): 140 NaCl, 4 KCl, 1 MgCl2, 2 CaCl2, 10 HEPES (pH 7.4 adjusted with NaOH). When intracellular Ca2+ release was assayed, Ca2+-free solution was used. This Ca2+-free solution was the same as the extracellular bath solution mentioned above except that Ca2+ was omitted and 20 μM EGTA was supplemented. Cells were alternately excited with 340 and 380 nm using an optical filter changer (Lambda 10–2, Sutter Instruments). Emission was collected at 500 nm and images were captured using a CCD camera (CoolSnap HQ2, Photometrics, Tucson, AZ) linked to an inverted Nikon TE 2000-U microscope. Images were analyzed with MAG Biosystems Software (Sante Fe, MN). All imaging experiments were performed at room temperature (25 °C).
Electrophysiology
Electrophysiological experiments were performed as previously reported [9]. Cells were voltage-clamped in the whole-cell configuration. Thin-walled borosilicate glass tubes (OD 1.5 mm, ID 1.10 mm, Sutter Instrument, Novato, CA) were pulled with a micropipette puller (P-87, Sutter Instrument), and then heat polished by a microforge (Narishige Instruments, Inc., Sarasota, FL). The typical pipette resistance filled with intracellular solution, containing (mM): 140 KCl, 1 MgCl2, 1 EGTA, 10 HEPES, and 5 MgATP (pH 7.25 adjusted with KOH), was 4–6 MΩ. The bath solution contained (mM): 140 NaCl, 4 KCl, 1 MgCl2, 2 CaCl2, 10 HEPES (pH 7.4 adjusted with NaOH). The currents were recorded using an EPC-10 amplifier with Pulse 8.60 acquisition software and analyzed by Pulsefit 8.60 software (HEKA Electronik, Lambrecht, Germany). Data were filtered at 2 kHz and sampled at 10 kHz. After a whole-cell configuration was established, the cells were held at −70 mV, and subject to depolarization to trigger outward Kv currents. Membrane potential was measured using the current-clamp mode. All experiments were performed at room temperature (25 °C).
Statistical analysis
Data are presented as mean ± SEM. The unpaired Student t-test was used to compare two groups. A value of p < 0.05 was considered to represent a significant difference.
Results
Depolarization of the astrocyte triggered outward K+ currents (Fig. 1). Addition of 20 mM TEA, an inhibitor of delayed rectifier Kv channels, suppressed the currents substantially. Further addition of TEA did not increase the extent of inhibition (not shown). The TEA-resistant currents had a fast inactivation typical of A-type currents, which could be inhibited by 30 μM quinidine. 4-Aminopyridine (4-AP), a classical A-type Kv channel blocker, was not used since it caused [Ca2+]i elevation (not shown), which would interfere in Ca2+ imaging experiments. It is believed that the outward currents observed were Kv currents and were unlikely to be ATP-sensitive K+ (KATP) currents since the pipette solution contained 5 mM ATP to inhibit KATP channels (if any). These currents were also unlikely to be Ca2+-activated K+ channels because small-conductance Ca2+-activated K+ channels and intermediate-conductance Ca2+-activated K+ channels are non-voltage activated, while large-conductance Ca2+-activated K+ (BK) channels are voltage-activated but could only be activated by 1–10 μM cytosolic Ca2+. The pipette solution was Ca2+-free and contained 1 mM EGTA, thus the cytosolic Ca2+ level would be too low to have any effect on BK channels.
Fig. 1.
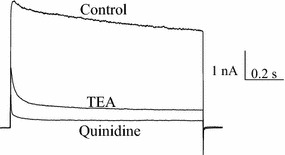
TEA-sensitive delayed rectifier and quinidine-sensitive A-type K+ currents were observed in astrocytes. A +70 mV depolarization elicited an outward Kv current; addition of 20 mM TEA inhibited delayed rectifier current, followed by inhibition of A-currents by 30 μM quinidine. Similar results were obtained in 5 more experiments
We hypothesized that Kv channel activities could regulate Ca2+ influx. The latter was triggered by Ca2+ store depletion by CPA, a sarcoplasmic/endoplasmic reticulum Ca2+-ATPase (SERCA) inhibitor. Such store-operated Ca2+ entry has been known to be important in astrocyte physiology [10]. In astrocytes bathed in Ca2+-free solution, CPA was added to cause Ca2+ store emptying; the latter procedure was performed to separate Ca2+ release from the subsequent Ca2+ influx after Ca2+ replenishment (Fig. 2a). Ca2+ influx was substantially diminished in the group with TEA and quinidine pre-incubation (Fig. 2b). The averaged results indicate that whilst Ca2+ release was comparable between the control and blocker-treated group (Fig. 2c), Ca2+ influx was significantly smaller (different from control group 56 s after Ca2+ replenishment; p < 0.05) in the blocker-treated group (Fig. 2d). DMSO was used to dissolve CPA. As a control, after cells were treated with DMSO (Fig. 2e), Ca2+ was replenished, and there was a small degree of Ca2+ influx (Fig. 2f). The reason was possibly that the cells had been bathed in a Ca2+-free solution supplemented with 20 mM EGTA for 7–8 min before Ca2+ replenishment. Thus, it is possible that this condition inevitably caused a small degree of Ca2+ store emptiness which could result in a very mild store-operated Ca2+ entry (compared to the large, fully-fledged CPA-triggered store-operated Ca2+ entry shown in Fig. 2d).
Fig. 2.
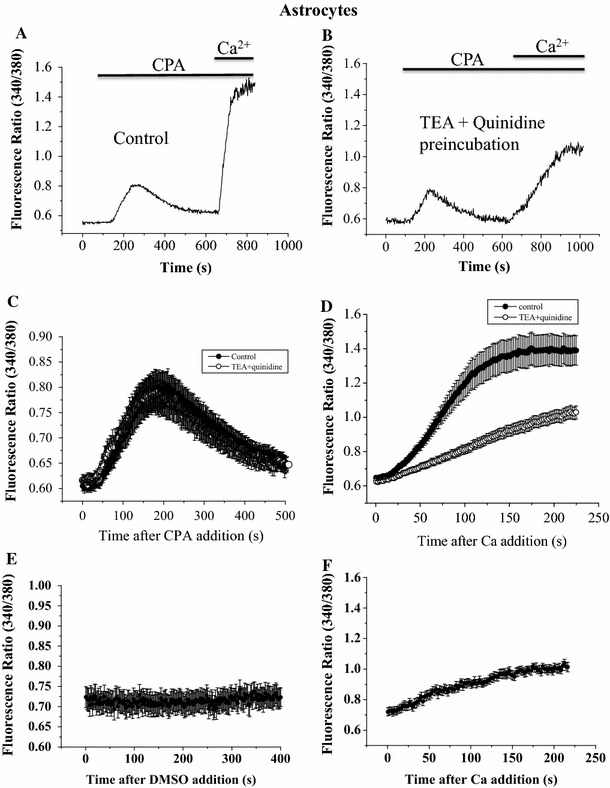
Block of astrocyte Kv channels by TEA and quinidine suppressed CPA-triggered Ca2+ influx. [Ca2+]i was measured using fura-2 as dye and quantified as fluorescence ratio. Astrocytes were bathed in Ca2+-free solution in the absence (a) or presence (b) of 20 mM TEA plus 30 μM quinidine; they were then exposed to 50 μM CPA followed by replenishment of 2 mM CaCl2. Quantification of the Ca2+ release component (c) and the Ca2+ influx component (d) TEA + quinidine group was different from control group 56 s after Ca2+ replenishment (p < 0.05). With DMSO treatment, there was no Ca2+ release (e) but a small degree of Ca2+ influx upon Ca2+ replenishment (f). Results are mean ± SEM of 15–24 cells from five separate experiments
BK channels are voltage-activated and could be inhibited by TEA. To rule out the possibility of BK channel involvement in regulating Ca2+ influx, we examined whether charybdotoxin (BK channel blocker) had an effect on CPA-triggered store-operated Ca2+ entry. After CPA-induced Ca2+ release (Fig. 3a), Ca2+ entry was not inhibited in the presence of charybdotoxin (Fig. 3b). Thus, BK channels (if any) did not appear to play a role in regulating Ca2+ entry.
Fig. 3.
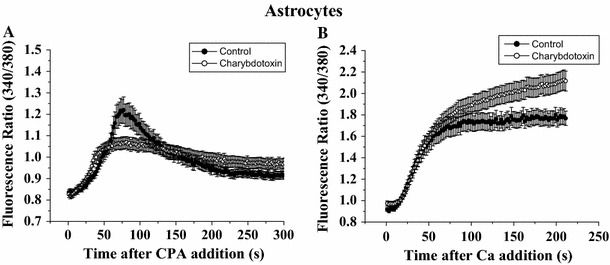
Charybdotoxin did not suppress CPA-triggered Ca2+ influx in astrocytes. Astrocytes were bathed in Ca2+-free solution in the absence or presence of a BKCa channel blocker charybdotoxin (100 nM); they were then exposed to 50 μM CPA followed by replenishment of 2 mM CaCl2. Quantification of the Ca2+ release component (a) and the Ca2+ influx component (b). Results are mean ± SEM of 26–56 cells from 3–5 separate experiments
We next investigated whether A-type channel or delayed rectifiers regulated CPA-triggered Ca2+ signaling. Using the same protocol as in Fig. 2, it was found that CPA-induced Ca2+ release was comparable in the control and blocker-treated groups: Ca2+ influx in the TEA group (Fig. 4a, b) or quinidine group (Fig. 4c, d) was smaller than that in the control groups but the difference did not reach statistical significance.
Fig. 4.
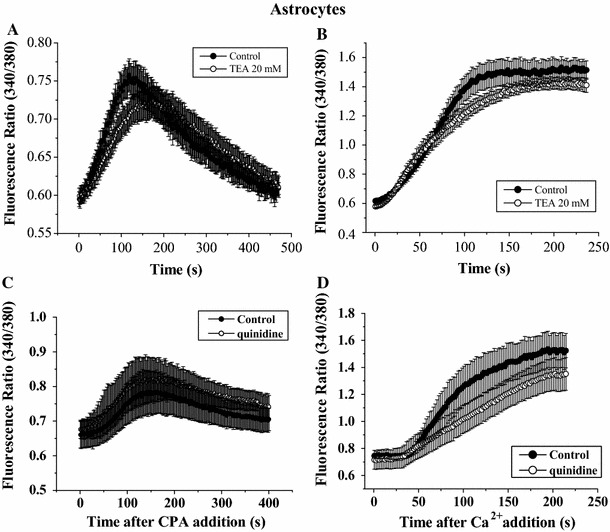
TEA or quinidine alone did not significantly affect CPA-triggered Ca2+ influx in astrocytes. Astrocytes were bathed in Ca2+-free solution in the absence or presence of a Kv channel blocker (20 mM TEA or 30 μM quinidine); they were then exposed to 50 μM CPA followed by replenishment of 2 mM CaCl2. Quantification of the Ca2+ release component (a) and the Ca2+ influx component (b) in the absence or presence of 20 mM TEA. Quantification of the Ca2+ release component (c) and the Ca2+ influx component (d) in the absence or presence of 30 μM quinidine. Results are mean ± SEM of 23–36 cells from 4 separate experiments
To rule out the possibility that the reduction of Ca2+ influx in the blocker-treated group (Fig. 2) was simply due to the inhibition of store-operated Ca2+ entry by TEA and quinidine, we examined CPA-triggered Ca2+ influx in H1355 cells, which are completely devoid of Kv channels [11]. There was no significant difference in CPA-induced Ca2+ release and Ca2+ influx between the control and blocker-treated groups (Fig. 5). Thus, it is unlikely that TEA and quinidine directly suppressed store-operated Ca2+ entry. Nevertheless, it is important to note that H1355 cells and astrocytes may have different store-operated Ca2+ channels.
Fig. 5.
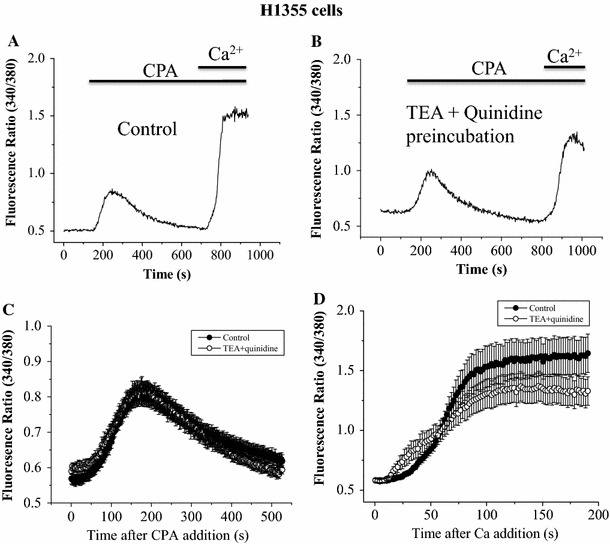
TEA and quinidine did not affect CPA-triggered Ca2+ influx in H1355 cells. H1355 cells were bathed in Ca2+-free solution in the absence (a) or presence (b) of 20 mM TEA plus 30 μM quinidine; they were then exposed to 50 μM CPA followed by replenishment of 2 mM CaCl2. Quantification of the Ca2+ release component (c) and the Ca2+ influx component (d; no significant difference between TEA + quinidine group and control group). Results are mean ± SEM of 13–14 cells from 3 separate experiments
We then examined how Kv channel activities could affect membrane potential changes in astrocytes. Since astrocytes do not naturally generate action potentials, we deployed the current-injection protocol [6, 12]. Injection of current produced a voltage overshoot, which partially repolarized after the peak (Fig. 6a). Application of TEA (20 mM) and quinidine (30 μM) in combination allowed the membrane potential to be raised to a much higher level. Figure 6b, c show the effects of sequential addition of blockers on voltage overshoot: effects of either blocker alone were smaller than those by both blockers in combination. In H1355 cells (devoid of Kv channels), injection of current produced a large voltage overshoot (without repolarization); adding both blockers did not affect such overshoot (Fig. 6d). These results suggest that Kv channels are important in dampening membrane potential changes in astrocytes.
Fig. 6.
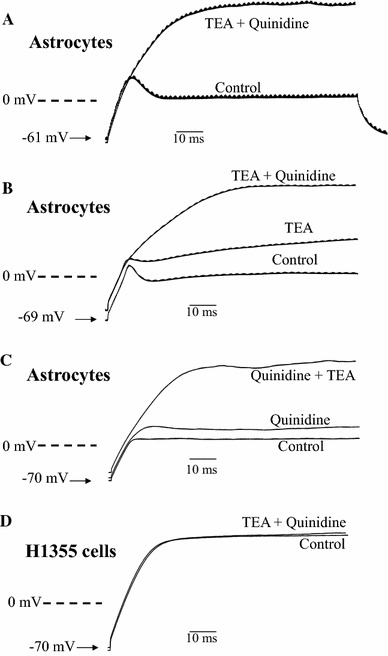
Astrocyte Kv channels regulate repolarization. a–c In astrocytes, a 3-nA current injection elicited a voltage overshoot, followed by a partial repolarization. Addition of 20 mM TEA plus 30 μM quinidine (a), 20 mM TEA followed by addition of 30 μM quinidine (b), or 30 μM quinidine followed by addition of 20 mM TEA (c), dramatically diminished the extent of repolarization. d In H1355 cells, overshoot elicited by a 3-nA current injection was not affected by 20 mM TEA plus 30 μM quinidine. Similar results were obtained in 5 more experiments
Discussion
Our data that astrocytes possessed A-type and delayed rectifier types of Kv channels (Fig. 1) are in accord with a previous report [2]. We here provide the first report that blocking both types of Kv channels by TEA and quinidine could substantially suppress store-operated Ca2+ influx. The latter Ca2+ entry has been known to be important in astrocyte physiology [10]. Our data could be interpreted in the following manner: Ca2+ influx could cause depolarization, which would trigger Kv channel opening. The latter would allow K+ efflux, thus curbing the degree of depolarization. Since in the non-excitable astrocyte, depolarization would indeed decrease the electrochemical driving force for Ca2+ influx, the curbing of depolarization by Kv channels is favorable for Ca2+ influx. Blocking the Kv channels by both TEA (delayed rectifier Kv channels blocker) and quinidine (A-type Kv channel blocker) would allow the astrocytes to depolarize more (see also Fig. 5a), a situation that limits Ca2+ entry (results in Fig. 2). It is noteworthy that TEA or quinidine alone did not significantly affect CPA-induced Ca2+ influx in astrocytes (Fig. 4). This is consistent with the observation that TEA or quinidine alone only slightly enhanced voltage overshoot, while these two drugs in combination produced a much more prominent effect on voltage overshoot (Fig. 6b, c). These results suggest that both A-type and delayed rectifier Kv channels are needed to cause significant regulation of membrane potential and thus Ca2+ influx.
In experiments deploying current injection-voltage overshoot protocol, 4-AP-sensitive Kv channels have been implicated in the regulation of membrane potential in spinal cord and hippocampal astrocytes [6, 12]. However, whether Kv channel activities could modulate Ca2+ signaling was unexplored. In this work, we showed that both A-type and delayed rectifier Kv channels were involved in regulating membrane potential and Ca2+ influx.
In chondrogenic cells (non-excitable cells), the amplitude and frequency of spontaneous Ca2+ oscillations are both inhibited by 10 mM TEA, which suppresses the chondrocyte Kv currents by 73 % [13]. This work also shows that TEA could inhibit chondrocyte proliferation and cartilage production. Pharmacological block of Kv1.3 in platelets has also been shown to inhibit store-operated Ca2+ influx and platelet count [14]. These works, together with the present report, suggest Kv channels could participate in regulating Ca2+ signaling and cell fate in non-excitable cells.
In conclusion, A-type and delayed rectifier Kv channels regulate membrane potential and thus Ca2+ influx in astrocytes. This bears the implication that Kv channels could modulate astrocyte “excitability” and gliotransmitter release.
Acknowledgments
YML would like to thank the Ministry of Science and Technology, Taiwan, for funding (NSC 101-2320-B-039-046; 103-2320-B-039 -035-). KCW would like to thank Eda Hospital and I-Shou University for support (EDAHP 103019).
Conflict of interest
The authors declare that they have no conflict of interest.
Contributor Information
Paul Chan, Phone: 886-2-29307930, Email: chanpaul@w.tmu.edu.tw.
Yuk-Man Leung, Phone: 886-4-22053366, Email: ymleung@mail.cmu.edu.tw.
References
- 1.Ransom BR, Ransom CB. Astrocytes: multitalented stars of the central nervous system. Methods Mol Biol. 2012;814:3–7. doi: 10.1007/978-1-61779-452-0_1. [DOI] [PubMed] [Google Scholar]
- 2.Olsen M. Examining potassium channel function in astrocytes. Methods Mol Biol. 2012;814:265–281. doi: 10.1007/978-1-61779-452-0_18. [DOI] [PubMed] [Google Scholar]
- 3.Perea G, Araque A. Glial calcium signaling and neuron-glia communication. Cell Calcium. 2005;38:375–382. doi: 10.1016/j.ceca.2005.06.015. [DOI] [PubMed] [Google Scholar]
- 4.Halassa MM, Fellin T, Haydon PG. Tripartite synapses: roles for astrocytic purines in the control of synaptic physiology and behavior. Neuropharmacology. 2009;57:343–346. doi: 10.1016/j.neuropharm.2009.06.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Parpura V, Verkhratsky A. The astrocyte excitability brief: from receptors to gliotransmission. Neurochem Int. 2012;61:610–621. doi: 10.1016/j.neuint.2011.12.001. [DOI] [PubMed] [Google Scholar]
- 6.Bordey A, Sontheimer H. Differential inhibition of glial K+ currents by 4-AP. J Neurophysiol. 1999;82:3476–3487. doi: 10.1152/jn.1999.82.6.3476. [DOI] [PubMed] [Google Scholar]
- 7.Lu DY, Yu WH, Yeh WL, Tang CH, Leung YM, Wong KL, Chen YF, Lai CH, Fu WM. Hypoxia-induced matrix metalloproteinase-13 expression in astrocytes enhances permeability of brain endothelial cells. J Cell Physiol. 2009;220:163–173. doi: 10.1002/jcp.21746. [DOI] [PubMed] [Google Scholar]
- 8.Leung YM, Huang CF, Chao CC, Lu DY, Kuo CS, Cheng TH, Chang LY, Chou CH. Voltage-gated K+ channels play a role in cAMP-stimulated neuritogenesis in mouse Neuro-2A cells. J Cell Physiol. 2011;226:1090–1098. doi: 10.1002/jcp.22430. [DOI] [PubMed] [Google Scholar]
- 9.Chou CH, Gong CL, Chao CC, Lin CH, Kwan CY, Hsieh CL, Leung YM. Rhynchophylline from Uncaria rhynchophylla functionally turns delayed rectifiers into A-type K+ channels. J Nat Prod. 2009;72:830–834. doi: 10.1021/np800729q. [DOI] [PubMed] [Google Scholar]
- 10.Verkhratsky A, Rodríguez JJ, Parpura V. Calcium signalling in astroglia. Mol Cell Endocrinol. 2012;353:45–56. doi: 10.1016/j.mce.2011.08.039. [DOI] [PubMed] [Google Scholar]
- 11.Leung YM, Wong KL, Lin CH, Chao CC, Chou CH, Chang LY, Chen SW, Cheng TH, Kuo YH. Dependence of 6-acetoxy-7-hydroxyroyleanone block of Kv1.2 channels on C-type inactivation gating. Cell Mol Life Sci. 2010;67:147–156. doi: 10.1007/s00018-009-0178-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Bekar LK, Loewen ME, Cao K, Sun X, Leis J, Wang R, Forsyth GW, Walz W. Complex expression and localization of inactivating Kv channels in cultured hippocampal astrocytes. J Neurophysiol. 2005;93:1699–1709. doi: 10.1152/jn.00850.2004. [DOI] [PubMed] [Google Scholar]
- 13.Varga Z, Juhász T, Matta C, Fodor J, Katona É, Bartok A, Oláh T, Sebe A, Csernoch L, Panyi G, Zákány R. Switch of voltage-gated K+ channel expression in the plasma membrane of chondrogenic cells affects cytosolic Ca2 + -oscillations and cartilage formation. PLoS ONE. 2011;6:e27957. doi: 10.1371/journal.pone.0027957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.McCloskey C, Jones S, Amisten S, Snowden RT, Kaczmarek LK, Erlinge D, Goodall AH, Forsythe ID, Mahaut-Smith MP. Kv1.3 is the exclusive voltage-gated K+ channel of platelets and megakaryocytes: roles in membrane potential, Ca2 + signalling and platelet count. J Physiol. 2010;588:1399–1406. doi: 10.1113/jphysiol.2010.188136. [DOI] [PMC free article] [PubMed] [Google Scholar]


