Abstract
The aim of this study was to examine gastric motility and blood flow during nociceptive hypertonic saline injections (HS) in paraspinal muscles of urethane-anaesthetised rats. Gastric pressure was not affected by HS in intact or vagotomised conditions. After cervical spinalisation, it was decreased by injections at T13 or L6 but not T2. Moreover, HS injections at T13 produced greater gastric pressure decreases compared with L6 and T2 and increased gastric sympathetic nerve activity. Blood pressure and gastric blood flow were decreased by T13 injections in spinal cord intact but not spinalised rats. Besides, isotonic saline injections (non-nociceptive) produced non-significant or marginal effects. These results indicate that gastric motility is decreased by nociceptive input from paraspinal muscles in spinalised rats through activation of the gastric sympathetic nerve. Although gastric blood flow was also decreased by nociceptive stimulation at T13 in spinal cord intact rats, these changes seem to depend on blood pressure.
Keywords: Muscle pain, Spinal, Back, Somato-autonomic reflexes, Gastric, Sympathetic
Introduction
Pain is associated with behavioural and physiological responses. These include autonomic responses such as reflex changes in gastric motility and cardiovascular functions. It is well known that somatic stimulation, including nociceptive muscle stimulation, can alter the activity of autonomic nerves and the function of their effector organs through so-called somato-autonomic reflexes [1]. Some of these reflexes, such as those elicited by limb stimulation, mainly rely on non-segmental pathways, while reflexes elicited by abdominal or back stimulation depend on both non-segmental and segmental pathways. The latter are sometimes masked by descending inhibitory pathways, as evidenced by spinal transection at the cervical level [1].
In anaesthetised rats, gastric motility is inhibited by nociceptive somatic stimulation applied to the abdomen by either pinching of the skin [2, 3] or needling of the skin and muscles [4], irrespectively of spinal cord transection [3, 4]. However, the reported effects of back stimulation (thoracic and lumbar regions) on gastric motility are variable. Indeed, gastric motility is not affected by needling [4], not affected or marginally inhibited by pinching [2], or clearly inhibited by capsaicin injections into interspinous tissues [5]. Therefore, gastric motility may be affected differently by back stimulation depending on the type of peripheral afferents that are activated and the stimulated tissue.
Acute back pain, including paraspinal muscle strain, is a common condition. Considering that back pain is highly prevalent in the general population [6–8] and that it may potentially affect the stomach through somato-sympathetic reflexes, it is essential to clarify the impact of paraspinal muscle nociception on gastric motility. To our knowledge, however, no study has investigated the modulation of gastric functions by paraspinal muscle stimulation alone to exclude the contribution of afferents from other tissues.
Besides, alteration of gastric blood flow (GBF) by back pain may also be clinically relevant since it plays an important role in tissue protection [9]. A few studies indicate that gastrointestinal blood flow is also affected by cutaneous heat stimulation on the abdomen through somato-autonomic reflexes [10, 11]. However, no study has examined the modulation of GBF by nociceptive input from back muscles. Importantly, muscle stimulation may have different effects on GBF compared to skin stimulation, considering the distinct changes in blood pressure that are associated with each stimulus. Indeed, nociceptive cutaneous stimulation usually increases blood pressure in the rat [1], while nociceptive stimulation of muscles is usually associated with decreases in blood pressure [12]. In turn, GBF may be affected by blood pressure changes passively or by reflex changes of gastric vasomotor nerves induced by somatic stimulation.
The aim of this study was to examine the impact of nociceptive input from paraspinal muscles on gastric motility and blood flow. Paraspinal muscles were stimulated with hypertonic saline (HS), a commonly used and potent chemical stimulus that reliably activates nociceptors and produces muscle pain in human [13–16]. We anticipated that the activation of paraspinal muscle nociceptors would be effective to alter gastric motility and blood flow in urethane-anaesthetised rats with an intact or a transected spinal cord through segmental sympathetic reflexes.
Methods
Animals
Three experiments were performed on 20 adult male Wistar rats (body weight 275–375 g) bred at the Tokyo Metropolitan Institute of Gerontology. The study was conducted with the approval of and in accordance with the guidelines for animal experimentation prepared by the Animal Care and Use Committee of the Tokyo Metropolitan Institute of Gerontology.
Experimental design
The study comprises three successive experiments to examine gastric motility, GBF, and gastric sympathetic nerve activity (GSNA) during nociceptive stimulation of paraspinal muscles. Nociceptive stimulation was carried out with a 20-μl injection of HS (6 %), while a 20-μl injection of isotonic saline (IS) (0.9 %) was used as a non-nociceptive control.
For experiment 1, HS and IS were injected in the T2, T13, or L6 left paraspinal muscles (n = 8). In this experiment, we specifically investigated whether gastric motility would be decreased by nociceptive paraspinal muscle stimulation and whether the reflexes were segmentally organised (stronger when the stimulation was delivered at T13) in central nervous system intact and spinalised conditions. We also wished to confirm the lack of contribution of the vagal nerve to these reflexes. Concurrently, we also examined how blood pressure would be affected by nociceptive input from the paraspinal muscles. Stimuli were delivered once for each condition and in random order for the two types of injections (HS or IS) at the three injection sites. The inter-stimulus interval was sufficiently long to allow recovery to baseline values (over 2 min). Once the series of six stimuli had been completed, vagotomy was performed bilaterally at the cervical level and a second series of six stimuli was delivered. Subsequently, rats were spinalised at the first cervical level, and a third series of six stimuli was delivered.
For experiment 2, GBF was examined in eight rats. For this experiment, HS and IS were injected at T13 in vagotomised and spinalised conditions only, based on the results from the first experiment. The order of nociceptive and non-nociceptive stimuli was counterbalanced and stimuli were delivered once for each condition. At the end of the experiment, GBF was measured during rapid blood withdrawal (0.7 ml) from the carotid artery in six of these rats to examine the potential passive effect of blood pressure changes on GBF.
For experiment 3, GSNA was monitored during HS and IS injections at T13 in vagotomised and spinalised conditions (n = 4) in order to examine the involvement of the gastric sympathetic nerve in gastric pressure and GBF changes. The order of nociceptive and non-nociceptive stimuli was counterbalanced and stimuli were delivered once for each condition.
General experimental conditions
Surgical procedures were initiated after animals had been deeply anaesthetised with urethane (1.1 g kg−1 i.p.). The right jugular vein was catheterised and additional urethane doses were administered by bolus i.v. injections (10 % of the initial dose) to maintain the depth of anaesthesia. In addition to stable systemic arterial blood pressure, the depth of anaesthesia was routinely confirmed by the absence of withdrawal (paw pinching) and corneal reflexes. Systemic mean arterial pressure (MAP) was monitored continuously from a cannula inserted into the right common carotid artery and connected to a pressure transducer (AT601-G, Nihon Kohden, Tokyo, Japan). Animals were artificially ventilated using a tracheal cannula (model SN-480-7, Shinano, Tokyo, Japan) and the end-tidal CO2 concentration was maintained to 3.0–3.5 % (Microcap, Oridion Medical, Jerusalem, Israel) by controlling the respiratory rate and tidal volume. Body temperature was monitored with a rectal probe and was maintained at 37.5 ± 0.5 °C with a body temperature control system (ATB-1100, Nihon Kohden, Tokyo, Japan). MAP as well as gastric pressure, GBF or gastric sympathetic nerve activity was then recorded continuously (Spike2, Cambridge Electronic Design, Cambridge, UK). Before beginning with the stimulation protocol, the depth of anaesthesia was confirmed and rats were immobilised with gallamine triethiodide (20 mg/kg, i.v.). For all experiments, rats rested for at least 20 min after vagotomy and spinalisation, and the signals were stable when the recordings started. At the end of the experiment, rats received a lethal dose of sodium pentobarbital.
Gastric pressure recording
Gastric pressure was measured with the balloon method [3] using a small balloon catheter (diameter around 1 cm). The balloon was inserted in the pyloric area of the stomach through a small duodenal incision, 3 cm away from the pylorus. Another catheter was placed in the duodenum to drain digestive secretions. After suturing the abdominal incision, the rat was placed in a prone position and the balloon catheter was connected to a pressure transducer (AT601-G, Nihon Kohden, Tokyo, Japan). The rat rested for approximately 1 h before any recording to allow recovery from surgery and stabilisation. At the beginning of the experiment, gastric pressure was increased between 100 and 150 mmH2O by injecting warm saline in the gastric balloon (between 0.1–0.3 ml) in order to induce typical peristaltic contractions. For quantification purposes, the mean gastric pressure was used (see data analyses below) because it provided a reliable and stable signal.
Gastric blood flow recording
Gastric blood flow was measured using laser Doppler flowmetry (ALF 2100, Advance, Tokyo). The probe (outer diameter, 1.0 mm) was gently placed on the anterior surface of the pyloric area of the stomach using a probe holder fixed to a static pole anchored to the surgical table. The holder could move freely in all movement planes to follow the small stomach movements induced by respiration and thus avoid large movement artefacts.
Gastric nerve recording
The gastric sympathetic nerve was exposed from opening in the posterior aspect of the abdominal muscles. A branch of the nerve was isolated by following the gastric branch of the coeliac trunk and was cut close to the stomach. A pool of warm paraffin oil was made and the proximal part of the cut end of the gastric nerve was placed on bipolar platinum-iridium hook electrodes. Multiunit activity was amplified (5,000 times), filtered (150–3,000 Hz), sampled at 20 kHz (Spike2 software, Cambridge Electronic Design, Cambridge, UK) and recorded on a personal computer for offline analyses.
Intramuscular saline injections
Small incisions were made on the skin of the back to insert 26-gauge needles into T2, T13 or L6 paraspinal muscles. Needles were connected to catheters and small syringes containing either IS (0.9 %) or HS (6 %). The needles were placed before any recording, including three for HS and three for IS (for experiment 1) or one for HS and one for IS (for experiment 2 and 3). After each series, needles were removed to avoid injury during vagotomy and spinalisation. Needles were then replaced in the same area for subsequent series. However, each site received only three injections at the most (either HS or IS), each of which was separated by at least 30 min. The 20-μl injections were performed manually over 10 s and always began after recovery from the needle insertion, when the signals were stable.
Data analyses and statistics
Data were analysed with Spike2 (Cambridge Electronic Design, Cambridge, UK). Gastric pressure and blood flow were smoothed with a time constant of 6 s, while mean arterial pressure was calculated with a time constant of 2 s. For gastric nerve recordings, multiunit activity was discriminated from background noise based on spike amplitude. The spike rate was then displayed continuously with 5-s time bins. For all measures, a 20-s baseline period was used to calculate the percent change induced by the stimulation (response peak − maximum or minimum).
All data are expressed as mean ± SEM. Statistical analyses were performed with Statistica v10.0 (Statsoft Inc., Tulsa, OK, USA). The effects of saline injections were assessed with paired t tests and repeated-measures ANOVA as needed. Fisher post hoc test was used to decompose significant effects. The significance threshold was set to p ≤ 0.05 for all analyses.
Results
Modulation of gastric motility by paraspinal muscle stimulation
An individual example of gastric pressure changes is shown in Fig. 1a. In this example of the T13 HS injection, gastric pressure did not show a clear response in the intact and vagotomised conditions but was robustly decreased after spinalisation. The response peaked within the first minute followed by a slow recovery to the baseline value after 2–3 min, but sometimes slightly later, as in this example. The peak of gastric pressure changes induced by paraspinal muscle stimulation (IS and HS injections) was measured within the first minute post-stimulus and was compared with baseline for each condition (intact, vagotomised and spinalised) and each region (T2, T13 and L6).
Fig. 1.
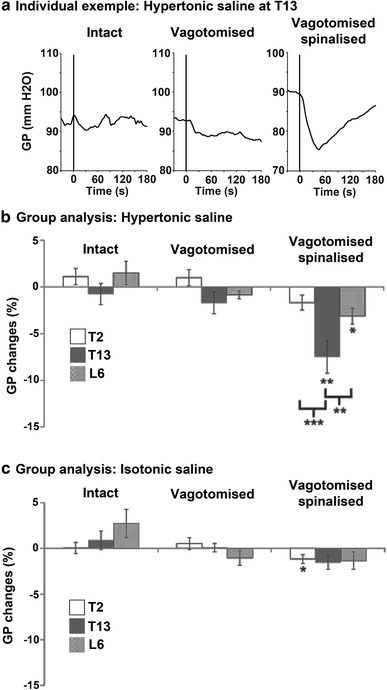
Modulation of gastric motility by nociceptive input from paraspinal muscles. a Individual example of mean gastric pressure (6-s smoothing) recordings in intact, vagotomised and spinalised conditions for the intramuscular HS injection at T13. Note that spinalisation was always performed after vagotomy so spinalised rats are also vagotomised. The vertical lines indicate the beginning of the HS injection that lasted approximately 10 s. Gastric pressure was not affected by HS in the intact and vagotomised conditions but was decreased after spinalisation. Group analyses (paired t tests, n = 8) are presented for HS (b) and for IS (c) injected in the paraspinal muscles of three vertebral regions, including T2, T13 and L6. *p < 0.05, **p < 0.01, ***p < 0.001
The group analysis of gastric pressure changes induced by HS injections is presented in Fig. 1b. Paired t tests indicated that gastric pressure was not affected by HS compared with baseline in intact conditions or after vagotomy (all p > 0.05). After spinalisation, however, gastric pressure decreased when HS was applied at T13 (−7.5 ± 1.8 %; p < 0.01) or L6 (−3.1 ± 0.9 %; p < 0.05), but not T2 (−1.7 ± 0.8 %; p > 0.05). To determine whether HS had a segmental effect (greater changes when injected at T13 compared with the other 2 regions), a one-way repeated measures ANOVA was conducted using the percent change in gastric pressure in spinalised conditions for the three regions. This analysis confirmed that the effect of HS was different between regions (main effect: p < 0.01). As expected, Fisher's post hoc test revealed a greater decrease in gastric pressure for T13 compared with L6 (p < 0.01) and T2 (p < 0.001).
As for the group analysis of gastric pressure changes induced by IS injections (see Fig. 1c), paired t tests indicated that intramuscular IS injection did not produce any significant change in gastric pressure compared with baseline for all conditions and all regions (p > 0.05) except for T2, for which a negligible but significant decrease was observed after spinalisation (−1.2 ± 0.5 %; p < 0.05).
Modulation of mean arterial pressure by paraspinal muscle stimulation
An individual example of MAP changes is shown in Fig. 2a. In this example of the T13 HS injection, MAP was strongly decreased in the intact and vagotomised conditions but not after spinalisation. The response peaked within 30 s and MAP recovered to the baseline value within 2 min. The peak of MAP changes induced by paraspinal muscle stimulation (IS and HS injections) was measured within the first minute post-stimulus and was compared with baseline for each condition (intact, vagotomised and spinalised) and each region (T2, T13 and L6).
Fig. 2.
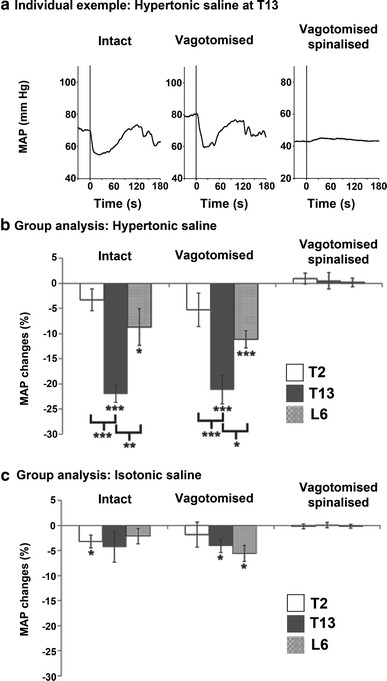
Modulation of blood pressure by nociceptive input from paraspinal muscles. a Individual example of mean arterial pressure (MAP) in intact, vagotomised and spinalised conditions for the intramuscular HS injection at T13. Note that spinalisation was always performed after vagotomy so spinalised rats are also vagotomised. The vertical lines indicate the beginning of the HS injection that lasted approximately 10 s. MAP was strongly decreased in the intact and vagotomised conditions but not after spinalisation. Group analyses (paired t tests, n = 8) are presented for HS (b) and for IS (c) injected in the paraspinal muscles of three regions, including T2, T13 and L6. *p < 0.05, **p < 0.01, ***p < 0.001
The group analysis of MAP changes induced by HS injections is presented in Fig. 2b. Paired t tests indicated that intramuscular HS injection strongly decreased MAP compared with baseline for T13 and L6 in intact conditions (−22.0 ± 1.7 %; p < 0.001 and −8.7 ± 3.6 %; p < 0.05, respectively) and in vagotomised conditions (−21.1 ± 2.8 %; p < 0.001 and −11.1 ± 1.7 %; p < 0.001, respectively). The response was abolished by spinalisation (both p > 0.05). No significant change was observed for T2 (all p > 0.1). A one-way repeated-measures ANOVA indicated that MAP responses induced by the HS injection differed across regions in intact conditions (main effect: p < 0.001) and after vagotomy (main effect: p < 0.01). Fisher's post hoc test revealed a greater decrease in MAP for T13 compared with L6 and T2 in intact conditions (p < 0.01 and p < 0.001, respectively) and after vagotomy (p < 0.05 and p < 0.001, respectively).
As for the group analysis of MAP changes induced by IS injections (see Fig. 2c), paired t tests indicated that MAP was slightly but significantly decreased relative to baseline in the intact conditions for T2 (−3.2 ± 1.3 %; p < 0.05) and after vagotomy for T13 (−4.1 ± 1.3 %; p < 0.05) and L6 (−5.6 ± 1.6 %; p < 0.05). No significant change was observed for other conditions (p > 0.05).
Modulation of GBF by paraspinal muscle stimulation
In experiment 2, GBF changes induced by HS and IS injections were examined for T13 in vagotomised and spinalised rats. An individual example is shown in Fig. 3a. In this example, GBF was decreased by the T13 HS injection in vagotomised conditions, while it was increased in spinalised conditions. The response peaked within the first minute (decrease or increase) and usually recovered to baseline values within 3 min, as in this example. The peaks of GBF changes induced by paraspinal muscle stimulation (IS and HS injections) were measured within the first minute post-stimulus and were compared with baseline for both conditions (vagotomised and spinalised).
Fig. 3.
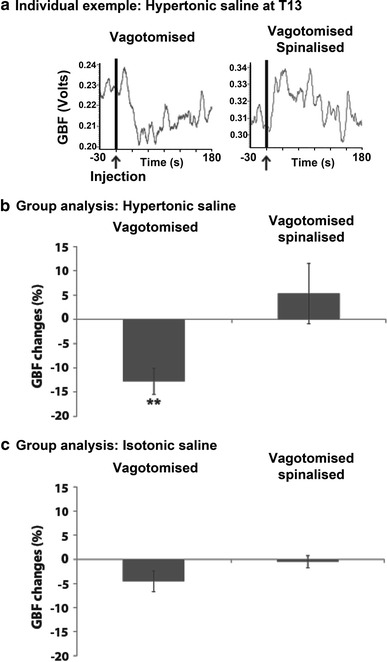
Modulation of gastric blood flow (GBF) by nociceptive input from T13 paraspinal muscles. a Individual example of GBF recordings in vagotomised and spinalised conditions. Note that spinalisation was always performed after vagotomy so spinalised rats are also vagotomised. The vertical lines indicate the beginning of the HS injection that lasted approximately 10 s. GBF was decreased by HS in vagotomised conditions but was increased after spinalisation. Group analyses (paired t tests, n = 8) are presented for HS (b) and for IS (c) injected in the paraspinal muscles of T13. **p < 0.01
The group analysis of GBF changes induced by HS injections is presented in Fig. 3b. Paired t tests indicated that intramuscular HS injection decreased GBF in vagotomised conditions (−12.8 ± 2.7 %; p < 0.01) but this reflex was abolished by spinalisation (p > 0.05), following which a trend toward an increase was observed. As for the group analysis of GBF changes induced by IS injections (see Fig. 3c), paired t tests indicated that IS did not significantly affect GBF compared with baseline in vagotomised or spinalised conditions (p > 0.05).
Mean arterial pressure changes recorded concurrently with GBF were also analysed with paired t tests to examine their potential contribution to GBF changes. HS decreased MAP compared to baseline in vagotomised conditions (−25.6 ± 2.7 %, p < 0.05). In spinalised conditions, HS did not significantly affect MAP compared to baseline, although it was slightly increased (6.3 ± 2.4 %, p > 0.05). Besides, IS did not significantly affect MAP compared with baseline in vagotomised (−9.2 ± 3.3 %, p > 0.05) or spinalised (0.0 ± 0.7 %, p > 0.05) conditions. Considering that HS produced similar effects on GBF and MAP in vagotomised conditions, MAP may contribute to GBF changes. Consistent with this, GBF changes were strongly associated with MAP changes in vagotomised conditions (Pearson’s correlation: r = 0.73, p < 0.05).
To confirm the possibility that GBF may be passively driven by MAP, the effect of rapid arterial blood withdrawal on GBF and MAP was examined (see Fig. 4). The withdrawal of 0.7 ml of blood from the carotid artery produced a decrease in MAP (−21.4 ± 3.5 %; p < 0.01) accompanied by a decrease of very similar amplitude in GBF (−21.6 ± 2.1 %; p < 0.001).
Fig. 4.
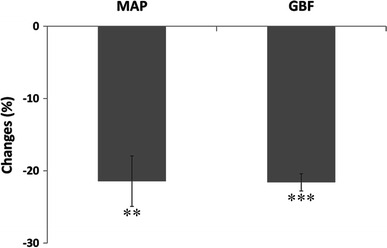
Passive changes in gastric blood flow (GBF) induced by withdrawal of arterial blood from the carotid artery. Withdrawal of 0.7 ml of arterial blood produced a 21 % decrease in MAP associated with a decrease of the same amplitude in GBF (paired t tests, n = 6). Note that the MAP changes reported here are comparable to MAP changes induced by HS injections at T13. **p < 0.01, ***p < 0.001
Activation of the gastric sympathetic nerve by stimulation of paraspinal muscles
Gastric sympathetic nerve activity was examined following paraspinal muscle stimulation with IS and HS injected at T13. An individual example is provided in Fig. 5a. In this example, GSNA was slightly decreased by HS in vagotomised conditions, while it was robustly increased after spinalisation. Similarly to gastric pressure, MAP and GBF, the response peaked within the first minute and recovered slowly to baseline. The peaks of GSNA induced by paraspinal muscle stimulation (IS and HS injections) were measured within the first minute post-stimulus and were compared with baseline for both conditions (vagotomised and spinalised).
Fig. 5.
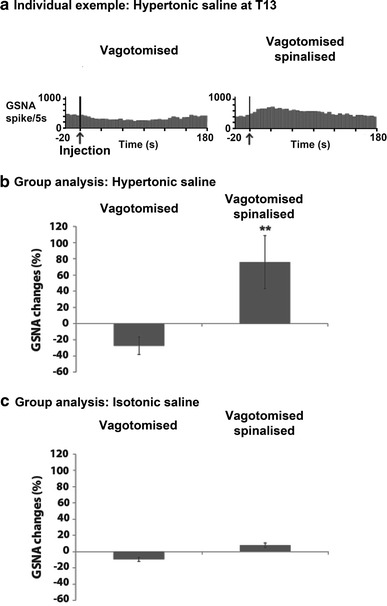
Modulation of gastric sympathetic nerve activity (GSNA) by nociceptive input from T13 paraspinal muscles. a Individual example of GSNA (spike rate) following HS injections at T13 in vagotomised and spinalised conditions. The vertical lines indicate the beginning of the HS injection that lasted approximately 10 s. HS tended to decrease GSNA in vagotomised conditions, while robustly increasing GSNA after spinalisation. Group analyses (paired t tests, n = 4) are presented for HS (b) and for IS (c) injected in the paraspinal muscles of T13. **p < 0.01
The group analysis of GSNA changes induced by HS injections is presented in Fig. 5b. Paired t tests indicated that intramuscular HS injection decreased GSNA compared with baseline in vagotomised conditions but this effect was not significant (−27.4 ± 10.8 %; p > 0.05). In contrast, gastric nerve activity showed a robust increase compared with baseline after spinalisation (75.8 ± 32.7 %; p < 0.01). As for the effect of IS injections (see Fig. 5c), GSNA was not significantly affected compared with baseline in either vagotomised or spinalised conditions (both p > 0.05).
Discussion
This is the first study that investigates somato-gastric reflexes induced by nociceptive input from paraspinal muscles selectively. Three novel findings emerged from the results. First, gastric motility was affected by nociceptive input from paraspinal muscles but only when the influence of descending inhibitory pathways was removed by spinal transection. Second, GBF was affected by nociceptive inputs from paraspinal muscles when the spinal cord was intact, but these changes seemed largely dependent on blood pressure. Third, HS injections produced a strong decrease in MAP, as expected. Intriguingly, however, the amplitude of this supraspinal response depended on the location of the stimulation.
Regulation of gastric motility
In the present study, gastric motility was decreased by HS injections in paraspinal muscles, but only after spinalisation. This response was segmentally organised, with a larger decrease observed for T13 compared with L6 or T2 injections, consistent with the central projections of T13 afferents [17, 18] and with the origin of preganglionic sympathetic neurons innervating the stomach [19]. Gastric motility was similarly unaffected by HS in the intact and vagotomised conditions, and the gastric pressure decrease induced by HS occurred in spinalised rats without connections to the vagal nerves. Therefore, gastric pressure changes induced by HS injections in paraspinal muscles were independent of vagal activity. These results are expected and consistent with previous studies showing the segmental organisation of somato-gastric reflexes [3–5]. In contrast to HS, the non-nociceptive IS injections at T13 did not produce any significant response, indicating that autonomic reflexes were elicited specifically by HS. Considering that HS activates group III and IV fibres and is one of the most potent nociceptive stimuli for muscles [14, 20, 21], this indicates that nociceptive stimulation of paraspinal muscles produces somato-gastric reflexes, while non-nociceptive stimulations are ineffective.
Besides, gastric motility was not affected by HS when the spinal cord was intact. This is consistent with previous studies using pinching of the skin along the vertebral column or needling in the back [2, 4], but not with a more recent study using capsaicin injections in interspinous tissues [5]. The discrepancies between studies may be due to several factors that seem to influence the response, including the location of the stimulation, the stimulus modality and the stimulus subtype (e.g. HS vs. capsaicin). Concerning the stimulus location, it appears that the same stimulation can produce different effects even when applied in regions innervated by the same segments of the spinal cord. For instance, pinching of the skin is either ineffective at modulating gastric motility (along the vertebral column), slightly inhibitory (more laterally in the back) or produces strong inhibition (on the abdomen) [2]. In humans, it was also observed that HS injection into the interspinous ligament produced pain that was longer in duration and greater in intensity compared to HS injection into the paraspinal muscles [22]. As for the stimulus modality, mechanical stimuli are ineffective at modulating gastric motility when applied along the vertebral column [2, 4], but capsaicin injected in the same region induces strong inhibitory effects [5]. Concerning the stimulus subtype, capsaicin and HS are both chemical agents that activate nociceptors, but produce different effects on gastric motility, as evidenced by a previous study [5] and the present results. Capsaicin strongly activates a subpopulation of group III and IV fibres containing TRPV1 channels [23–25]. HS also activates group III and IV fibres of the skeletal muscle [14, 20, 21], but it may activate subpopulations of fibres non-selectively, with or without TRPV1 channels. Thus, different responses may be observed with HS and capsaicin.
Alternatively, the effect of the different stimuli on blood pressure should be considered. In the study using capsaicin injections, one remarkable difference compared with the present study is that MAP was dramatically increased by capsaicin [5] as opposed to the robust decrease induced by HS. The drop in MAP induced by HS in the present study is consistent with the typical effect of deep structure stimulation [12]. In contrast, capsaicin is more consistent with the activation of nociceptors in superficial tissues [1]. As opposed to the present study, capsaicin injections were performed without removing the skin so it may be that subcutaneous tissues were also strongly stimulated in addition to the deeper interspinous structures [5]. Future experiments are therefore needed to clarify the mechanisms underlying the differences between studies by comparing HS and capsaicin injections in the same tissues and at the same location with peripheral afferent recordings.
In the present study, GSNA was recorded to confirm that it was responsible for the inhibitory effect of HS injections on gastric motility. Results indicate that GSNA was robustly increased by HS in spinalised conditions, which decreased gastric motility. This is consistent with the previously described mechanism of gastric motility inhibition by somatic stimuli, involving segmental activation of the GSNA by nociceptive input [3–5]. In contrast, GSNA was not significantly affected in spinal cord intact vagotomised rats, although there was a trend toward inhibition. This result suggests that segmental somato-GSNA reflex activation is masked by descending inhibitory pathways. Considering previous results showing different effects of pinching and needling to the abdomen vs. back, our result raises the possibility that descending inhibitory pathways may be preferentially activated by back stimulation rather than abdominal stimulation.
Regulation of gastric blood flow
In contrast to gastric motility, GBF was decreased in rats with an intact spinal cord by nociceptive inputs from T13 paraspinal muscles. It has been shown that nociceptive warming of the abdominal skin to 50 °C or above produced a vasoconstriction in the gastrointestinal tract in both rats with an intact central nervous system and rats with transected spinal cords [10]. However, the present GBF decrease observed in parallel with changes in systemic MAP was abolished by spinalisation, indicating that it is of supraspinal origin, similarly to the MAP response. Therefore, it appears to be a passive response induced by lower tissue perfusion. This is consistent with (1) the strong correlation between GBF and MAP changes following HS injections, (2) the almost identical decrease of GBF and MAP following rapid withdrawal of arterial blood in the carotid artery, and (3) HS stimulation that decreased GBF but did not activate (rather tended to inhibit) GSNA, which contains a population of gastric vasoconstrictor fibres [26]. A similar blood flow response in the mesenteric microvasculature was reported in a previous study showing that the blood flow decrease induced by electro-acupuncture applied to the back mainly depended on the systemic arterial pressure [27].
One potential mechanism of the hypotensive response is the inhibition of tonic activity in the rostral ventrolateral medulla (RVLM), which regulates the systemic vasomotor tone [28]. Accordingly, inhibition of the RVLM by HS injections may produce a withdrawal of premotor sympathetic activity, leading to a generalised deactivation of sympathetic vasomotor neurons in the spinal cord (see Fig. 6a). Consequently, this would lead to vasodilation and a depressor response. Besides, descending pathways also inhibit spinal reflexes involving the sympathetic motor neurons, preventing the inhibition of gastric motility by somato-sympathetic reflexes when the spinal cord is intact.
Fig. 6.
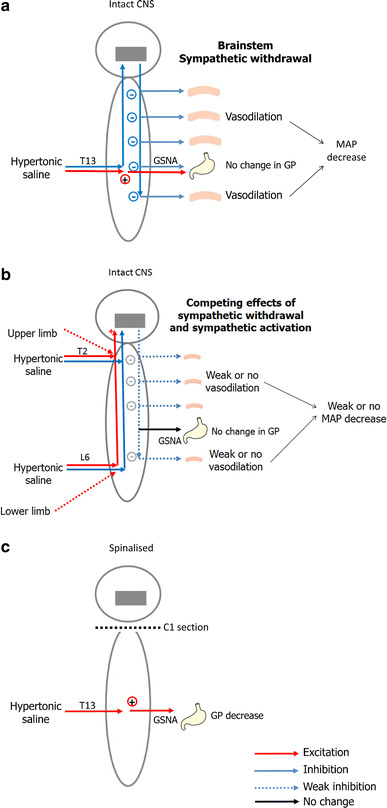
Schematic illustration of the proposed mechanisms underlying somato-autonomic reflexes induced by nociceptive stimulation of paraspinal muscles. a In intact conditions, stimulation of paraspinal muscles with hypertonic saline inhibits tonic sympathetic activity in the brainstem and activates descending inhibitory pathways. This produces a generalised deactivation of sympathetic vasomotor neurons of spinal origin, which in turn induces vasodilation and a depressor response. Besides, descending pathways also inhibit spinal reflexes involving the gastric sympathetic motor neurons, preventing the inhibition of gastric motility by somatic-sympathetic reflexes when the spinal cord is intact. b Nociceptive inputs from T2 and L6 project, in part, to spinal neurons that also receive inputs from the limbs. This partial convergence of back afferents on spinal neurons that receive limb afferents and project to pressor regions in the brainstem may trigger a pressor response. In turn, this response may compete with the depressor response induced by the inhibition of the tonic sympathetic activity in the brainstem. The resulting response, integrated in the brainstem, may then be smaller or abolished compared with the depressor response induced by the HS injection at T13, which nociceptors project to spinal segments devoid of neurons that receive limb afferent inputs. c In spinalised conditions, descending pathways are interrupted, leading to no change in blood pressure. However, the existing connection between somatic afferents from the back and preganglionic sympathetic neurons is disinhibited and allows somato-gastric reflexes to be evoked by hypertonic saline injections. It should be noted that vasodilation was not measured directly in this study, but was rather extrapolated from the GBF and MAP responses. Considering that a strong depressor response was produced by HS, it must be associated with some vasodilation, although it was not monitored. CNS central nervous system, GSNA gastric sympathetic nerve activity, MAP mean arterial pressure, GP gastric pressure
Differences in the amplitude of the MAP response depending on stimulus location
An intriguing finding of this study is the apparent segmental effect of HS injections on the MAP response in central nervous system intact conditions. Indeed, when injected at T13, HS produced greater MAP changes compared with T2 and L6. However, these responses were abolished by spinalisation, indicating their supraspinal origin. This may reflect a difference in the connectivity of nociceptors from different regions of the spine (see Fig. 6). For instance, nociceptive inputs from T2 and L6 project, in part, to spinal neurons that also receive inputs from the limbs. Therefore, partial convergence of back afferents on spinal neurons that receive limb afferents and project to pressor regions in the brain may trigger a pressor response. In turn, this response may compete with the depressor response induced by RVLM inhibition (see above). The resulting response, integrated in the RVLM, may then be smaller or abolished compared with the depressor response induced by the HS injection at T13, which nociceptors project to spinal segments devoid of neurons that receive limb afferent inputs. However, this remains speculative and future studies are needed to clarify the mechanisms of segment-dependant differences in the amplitude of MAP changes.
Speculations on clinical significance
Since gastric blood flow but not gastric motility was affected by nociceptive input from back muscles in rats with an intact central nervous system, we speculate that muscle pain from the back may alter gastric function possibly through a decrease in gastric blood flow in patients with an intact spinal cord. Besides, based on the results from the present study in spinalised rats, gastric motility may be altered in patients with a complete spinal cord injury presenting with a concurrent musculoskeletal disorder affecting their thoracic spine, although they do not feel or report pain. Consistent with this, it has been reported that patients with complete high cervical spinal cord injury may experience gastric dysmotility following an abdominal surgery, as a manifestation of active nociceptive processes and as a consequence of autonomic hyperreflexia [29]. Considering that the abdomen and the back share similar segmental innervation, a comparable surgery or lesion to back tissues may produce similar effects, based on the present findings.
Conclusion
In conclusion, nociceptive input from the thoraco-lumbar spine may not always produce somato-gastric reflexes when the spinal cord is intact. The conditions for which these reflexes may occur need to be clarified, but this study indicates that in the case of HS injected in the paraspinal muscles, descending pathways profoundly inhibit spinal reflexes that evoke sympathetic responses, resulting in no significant change in gastric motility. In addition, HS likely produces a withdrawal of premotor sympathetic activity in the brainstem. This leads to a generalised deactivation of sympathetic vasomotor neurons of spinal origin that produces a large depressor response associated with a decrease in gastric blood flow.
Acknowledgments
This study was funded by the Japan Society for the Promotion of Science, the Natural Sciences and Engineering Research Council of Canada and the “Fondation de Recherche en Chiropratique du Québec”. MP is also supported by a chair in pain neurophysiology from the Université du Québec à Trois-Rivières. The authors would also like to thank Dr. Sae Uchida for helpful discussions during the preparation of this manuscript.
Conflict of interest
The authors declare that they have no conflict of interest.
References
- 1.Sato A, Sato Y, Schmidt RF. The impact of somatosensory input on autonomic functions. Rev Physiol Biochem Pharmacol. 1997;130:1–328. doi: 10.1007/BFb0046598. [DOI] [PubMed] [Google Scholar]
- 2.Kametani H, Sato A, Sato Y, Ueki K. Reflex facilitation and inhibition of gastric motility from various skin areas in rats. In: Ito M, editor. Integrative control functions of the brain. Tokyo: Kondansha Scientific; 1978. pp. 285–287. [Google Scholar]
- 3.Kametani H, Sato A, Sato Y, Simpson A. Neural mechanisms of reflex facilitation and inhibition of gastric motility to stimulation of various skin areas in rats. J Physiol. 1979;294:407–418. doi: 10.1113/jphysiol.1979.sp012937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Sato A, Sato Y, Suzuki A, Uchida S. Neural mechanisms of the reflex inhibition and excitation of gastric motility elicited by acupuncture-like stimulation in anesthetized rats. Neurosci Res. 1993;18(1):53–62. doi: 10.1016/0168-0102(93)90105-Y. [DOI] [PubMed] [Google Scholar]
- 5.Budgell B, Suzuki A. Inhibition of gastric motility by noxious chemical stimulation of interspinous tissues in the rat. J Auton Nerv Syst. 2000;80(3):162–168. doi: 10.1016/S0165-1838(00)00100-4. [DOI] [PubMed] [Google Scholar]
- 6.Deyo RA, Mirza SK, Martin BI. Back pain prevalence and visit rates: estimates from US national surveys, 2002. Spine (Phila Pa 1976) 2006;31(23):2724–2727. doi: 10.1097/01.brs.0000244618.06877.cd. [DOI] [PubMed] [Google Scholar]
- 7.Yamada K, Matsudaira K, Takeshita K, Oka H, Hara N, and Takagi Y (2013) Prevalence of low back pain as the primary pain site and factors associated with low health-related quality of life in a large Japanese population: a pain-associated cross-sectional epidemiological survey. Mod Rheumatol (in press) [DOI] [PubMed]
- 8.Manchikanti L, Singh V, Datta S, Cohen S, Hirsch J. Comprehensive review of epidemiology, scope, and impact of spinal pain. Pain Physician. 2009;12(4):E35–E70. [PubMed] [Google Scholar]
- 9.Wallace JL. Prostaglandins, NSAIDs, and gastric mucosal protection: why doesn’t the stomach digest itself? Physiol Rev. 2008;88(4):1547–1565. doi: 10.1152/physrev.00004.2008. [DOI] [PubMed] [Google Scholar]
- 10.Kuntz A. Anatomic and physiologic properties of cutaneo-visceral vasomotor reflex arcs. J Neurophysiol. 1945;8:421–429. doi: 10.1152/jn.1945.8.6.421. [DOI] [PubMed] [Google Scholar]
- 11.Kuntz A, Haselwood LA. Circulatory reactions in the gastrointestinal tract elicited by localized cutaneous stimulation. Am Heart J. 1940;20:743–749. doi: 10.1016/S0002-8703(40)90533-6. [DOI] [Google Scholar]
- 12.Keay KA, Clement CI, Owler B, Depaulis A, Bandler R. Convergence of deep somatic and visceral nociceptive information onto a discrete ventrolateral midbrain periaqueductal gray region. Neuroscience. 1994;61(4):727–732. doi: 10.1016/0306-4522(94)90395-6. [DOI] [PubMed] [Google Scholar]
- 13.Steinbrocker O, Isenberg SA, Silver M, Neustadt D, Kuhn P, Schittone M. Observations on pain produced by injection of hypertonic saline into muscles and other supportive tissues. J Clin Invest. 1953;32(10):1045–1051. doi: 10.1172/JCI102815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Cairns BE, Svensson P, Wang K, Hupfeld S, Graven-Nielsen T, Sessle BJ, Berde CB, Arendt-Nielsen L. Activation of peripheral NMDA receptors contributes to human pain and rat afferent discharges evoked by injection of glutamate into the masseter muscle. J Neurophysiol. 2003;90(4):2098–2105. doi: 10.1152/jn.00353.2003. [DOI] [PubMed] [Google Scholar]
- 15.Cairns BE. Physiological properties of thin-fiber muscle afferents: excitation and modulatory effects. In: Graven-Nielsen T, Arendt-Nilsen L, Mense S, editors. Fundamentals of musculoskeletal pain. Seattle: IASP Press; 2008. pp. 19–32. [Google Scholar]
- 16.Kellgren JH. Observations on referred pain arising from muscle. Clin Sci. 1938;3:175–190. [Google Scholar]
- 17.Budgell B, Noda K, Sato A. Innervation of posterior structures in the lumbar spine of the rat. J Manip Physiol Ther. 1997;20(6):359–368. [PubMed] [Google Scholar]
- 18.Taguchi T, Hoheisel U, Mense S. Dorsal horn neurons having input from low back structures in rats. Pain. 2008;138(1):119–129. doi: 10.1016/j.pain.2007.11.015. [DOI] [PubMed] [Google Scholar]
- 19.Strack AM, Sawyer WB, Marubio LM, Loewy AD. Spinal origin of sympathetic preganglionic neurons in the rat. Brain Res. 1988;455(1):187–191. doi: 10.1016/0006-8993(88)90132-1. [DOI] [PubMed] [Google Scholar]
- 20.Kumazawa T, Mizumura K. Thin-fibre receptors responding to mechanical, chemical, and thermal stimulation in the skeletal muscle of the dog. J Physiol. 1977;273(1):179–194. doi: 10.1113/jphysiol.1977.sp012088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Paintal AS. Functional analysis of group III afferent fibres of mammalian muscles. J Physiol. 1960;152:250–270. doi: 10.1113/jphysiol.1960.sp006486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Tsao H, Tucker KJ, Coppieters MW, Hodges PW. Experimentally induced low back pain from hypertonic saline injections into lumbar interspinous ligament and erector spinae muscle. Pain. 2010;150(1):167–172. doi: 10.1016/j.pain.2010.04.023. [DOI] [PubMed] [Google Scholar]
- 23.Kaufman MP, Iwamoto GA, Longhurst JC, Mitchell JH. Effects of capsaicin and bradykinin on afferent fibers with ending in skeletal muscle. Circ Res. 1982;50(1):133–139. doi: 10.1161/01.RES.50.1.133. [DOI] [PubMed] [Google Scholar]
- 24.Hoheisel U, Reinöhl J, Unger T, Mense S. Acidic pH and capsaicin activate mechanosensitive group IV muscle receptors in the rat. Pain. 2004;110(1–2):149–157. doi: 10.1016/j.pain.2004.03.043. [DOI] [PubMed] [Google Scholar]
- 25.Caterina MJ, Schumacher MA, Tominaga M, Rosen TA, Levine JD, Julius D. The capsaicin receptor: a heat-activated ion channel in the pain pathway. Nature. 1997;389(6653):816–824. doi: 10.1038/39807. [DOI] [PubMed] [Google Scholar]
- 26.Furness JB, Costa M. The adrenergic innervation of the gastrointestinal tract. Ergeb Physiol. 1974;69:2–51. [PubMed] [Google Scholar]
- 27.Takagi K, Yamaguchi S, Ito M, Ohshima N. Effects of electroacupuncture stimulation applied to limb and back on mesenteric microvascular hemodynamics. Jpn J Physiol. 2005;55(3):191–203. doi: 10.2170/jjphysiol.R2115. [DOI] [PubMed] [Google Scholar]
- 28.Guyenet PG. The sympathetic control of blood pressure. Nat Rev Neurosci. 2006;7(5):335–346. doi: 10.1038/nrn1902. [DOI] [PubMed] [Google Scholar]
- 29.Pacheco MS, Garstang SV. Gastric dysmotility after abdominal surgery in persons with cervical spinal cord injury: a case series. J Spinal Cord Med. 2007;30(4):378–384. doi: 10.1080/10790268.2007.11771866. [DOI] [PMC free article] [PubMed] [Google Scholar]


