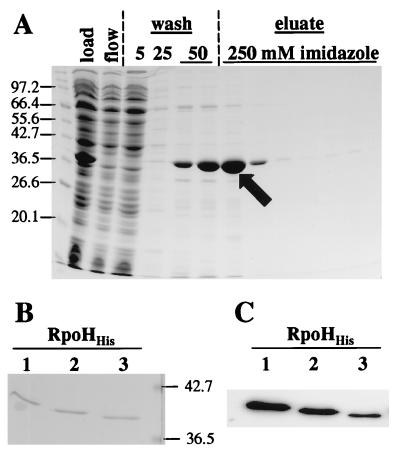
FIG. 4.
Purification of RpoH2His and immunodetection of B. japonicum RpoHHis proteins. (A) Aliquots of the RpoH2His supernatant fraction which was loaded onto the column (load), the flowthrough (flow), the different wash (wash), and the elution (eluate) fractions were separated on an SDS–12% polyacrylamide gel, and the proteins were stained with Coomassie blue. The imidazole concentration in the wash and elution buffers is indicated. The apparent molecular masses (in kilodaltons) of the marker proteins are indicated to the left of the gel. The arrow points to the protein fraction which was subsequently used for in vitro experiments. (B) Coomassie blue-stained SDS–12% polyacrylamide gel of 100 ng of each purified B. japonicum RpoHHis protein. The apparent molecular masses of two marker proteins are indicated to the right of the gel. (C) Immunodetection of purified B. japonicum RpoH proteins with anti-E. coli ς32 serum. For each protein, a 5-ng sample was subjected to Western blot analysis.