Figure 2. Patterns of Dose Escalation During Index Treatment Among All Patients (A) and Among Patients With CS (B).
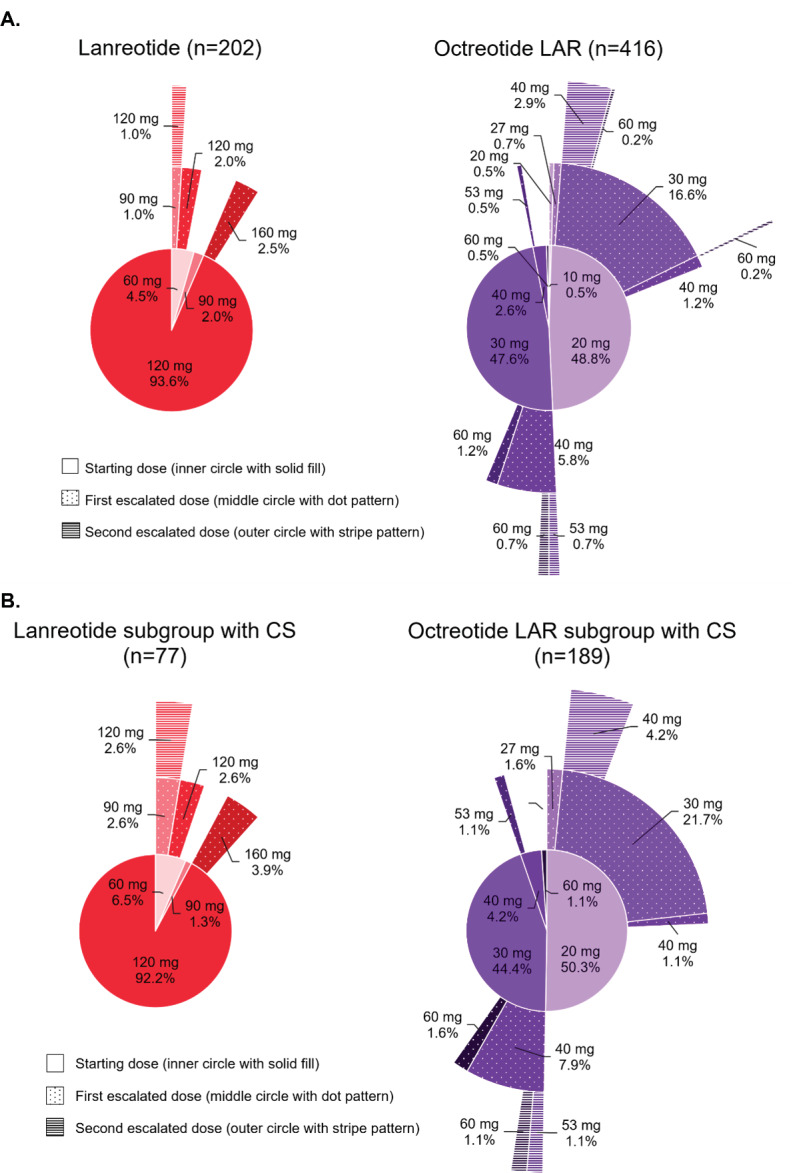
- Among all patients, doses were reported at treatment initiation, first escalation, and second escalation for 202, 11, and 2 lanreotide patients, and 416, 110, and 20 octreotide LAR patients, respectively.
- Among patients with CS, doses were reported at treatment initiation, first escalation, and second escalation for 77, 7, and 2 lanreotide patients, and 189, 66, and 12 octreotide LAR patients, respectively.
The inner, middle, and outer circles show the doses at initiation, after first escalation, and after second dose escalation during the index treatment. The percentages present the proportion of patients with the reported dose among (A) the overall cohorts (202 lanreotide and 416 octreotide LAR) and (B) the CS subgroups (77 lanreotide and 189 octreotide LAR). For octreotide LAR, non-standard 28-day doses were observed due to frequency-based dose escalations. Doses of 27 mg represented the patients who were on 20 mg every 28 days and increased frequency to every 21 days (20 mg/21*28 = 27 mg), while 53 mg represented the patients who were on 40 mg every 28 days and increased frequency to every 21 days (40 mg/21*28 = 53 mg).
Abbreviations: CS, carcinoid syndrome; LAR, long-acting release.
