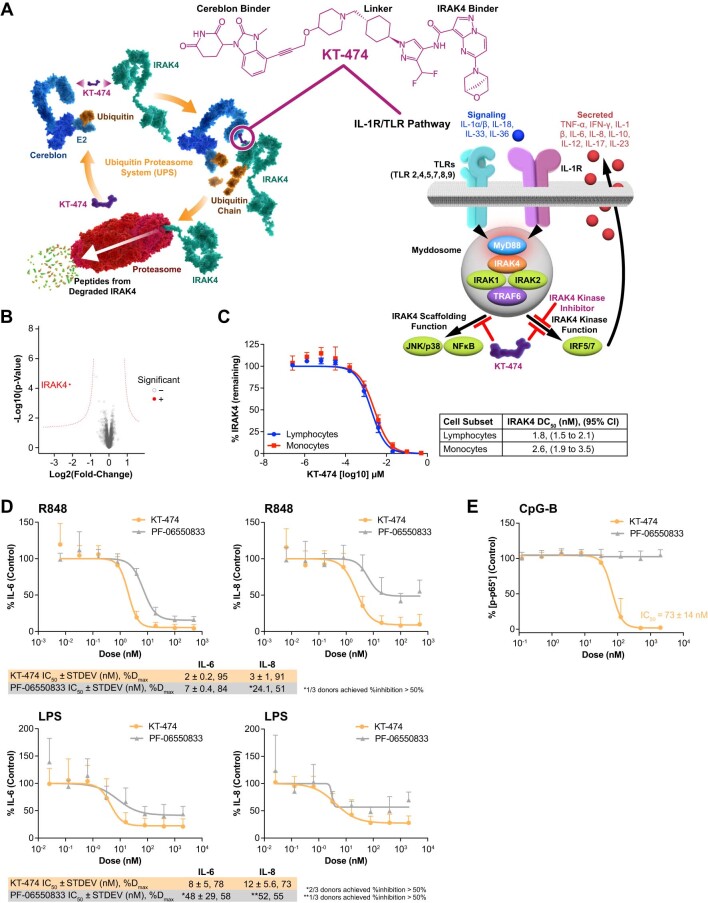
Extended Data Fig. 1. Identification of KT-474 (SAR444656) as a potent and selective degrader of IRAK4.
(a) KT-474 chemical structure, mechanism of action and potential advantage over IRAK4 kinase inhibitors on inhibition of TLR–/IL-1R–driven myddosome signaling through its effects on scaffolding and kinase functions of IRAK4. © 2023 Kymera Therapeutics, Inc. (b) Highly selective degradation of IRAK4 by KT-474 in human PBMCs determined by discovery proteomics. A paired statistical analysis was performed using linear models and significant degradation was determined by application of a weighted cutoff incorporating both significance and fold-change. (c) Potent degradation of IRAK4 by KT-474 in lymphocyte and monocyte subsets of PBMCs using N = 3 biologically independent donors over 1 experiment. (d) Superior inhibition of IL-6 and IL-8 production in R848- or LPS-stimulated PBMCs by KT-474 compared to the IRAK4 kinase inhibitor PF-06550833 using N = 3 biologically independent donors and 2 technical replicates over 1 experiment. (e) Inhibition of NF-kB activation (phospho-p65) in CpG-B stimulated B cells by KT-474 but not PF-06550833 using N = 5 biologically independent donors over 2 experiments. All data in (c) through (e) are graphed as mean values +/- STDEV. CI = confidence interval; IC50 = concentration of a drug needed to inhibit a biological process or response by 50%; IL = interleukin; LPS = lipopolysaccharide; PBMCs = peripheral blood mononuclear cells; R = receptor; STDEV = standard deviation; TLR = toll-like receptor.