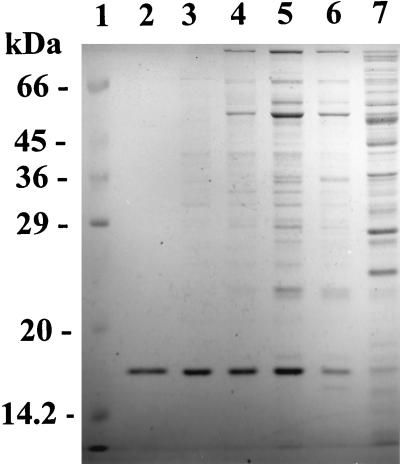
FIG. 1.
SDS–15% PAGE of 2-aminomuconate deaminase. Purified 2-aminomuconate deaminase (lane 2; 2 μg) was compared with the crude extract (lane 7; 40 μg), the DEAE fraction (lane 6; 20 μg), the Cu(II)-chelating fraction (lane 5; 20 μg), the gel filtration fraction (lane 4; 20 μg), the Hitrap-Q fraction (lane 3; 7 μg), and protein molecular mass standards (lane 1).