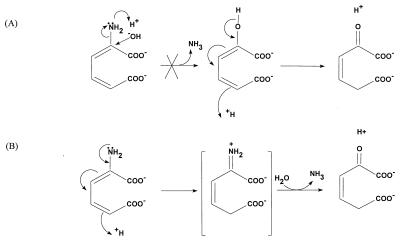
FIG. 3.
Alternative hypothetical mechanisms of deamination of 2-aminomuconate. (A) A hydroxyl group attacks the α-carbon of 2-aminomuconate to form 2-hydroxymuconate, which slowly tautomerizes to 4-oxalocrotonate (not observed). (B) A proton from the aqueous solution (spontaneous deamination) or from a proton donor (enzymatic catalysis) initiates tautomerization to form an imine intermediate which hydrolyzes spontaneously to 4-oxalocrotonate.