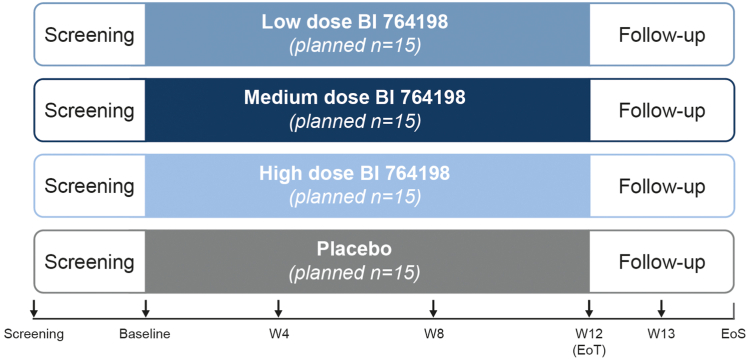
Figure 2.
Study design. Patients will be randomized to receive 1 of 3 doses of BI 764198 (low, medium, or high) or placebo for 12 weeks. Patients will enter a follow-up period at the end of the treatment period, with follow-up visits at days 7 (week 13) and 30 (EoS) after the end of treatment. Colored boxes represent the treatment period and arrows indicate visits where endpoint measurements will be taken. EoS, end of study; EoT, end of treatment; W, week.