Figure 3.
A common C-core conformation is induced by shared HCDR features of VH3-33 mAbs
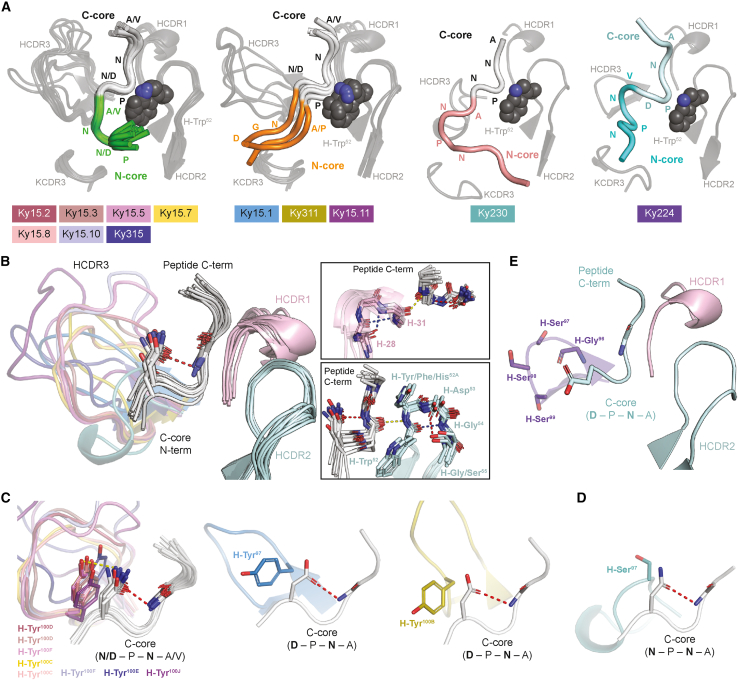
(A) Overview of N- and C-core motifs of different binding conformations. Common C-core conformations are colored in light gray, the unique C-core conformation induced by Ky224 is shown in pale cyan, and N-core motifs are shown in green, orange, salmon, and cyan. Positions of N- and C-core amino acids (aa) are labeled. Structures of mAbs exhibiting the same PfCSP recognition mode are superposed, with HCDRs and KCDR3s in dark gray cartoon representation and VH3-33 GL residue H-Trp52 shown as spheres. The legend below indicates which peptide conformation is induced by which mAb.
(B) C-core conformation of various peptides (gray) bound independently by 11 different mAbs. HCDR1s and HCDR2s are shown in light pink and pale cyan, respectively. Peptide C-terminal (C-term) and C-core N-terminal (N-term) ends are labeled, and residues facilitating the Asx turn are represented as sticks. Insets on the right highlight features of HCDR1 and HCDR2 that are shared between the 11 mAbs and stabilize the C-core conformation.
(C and D) HCDR3 Tyr (C) and Ser (D) residues supporting the C-core Asx turn through stacking interactions and HBs. Interacting residues are indicated in bold text and shown as sticks. Tyr residues in the same structural position and rotameric state are overlaid in a single panel.
(B–D) Red and blue dashed lines represent intrachain HBs mediated by side-chain atoms and main-chain atoms, respectively. Yellow dashed lines represent interchain HBs.
(E) C-core conformation of the NPDP peptide (pale cyan) bound by Ky224. HCDR1 and 2 are colored as in (B). HCDR3 residues are labeled and shown as sticks. The peptide C-term is labeled, and C-core residues corresponding to those mediating the Asx turn observed in other binding modes are represented as sticks and indicated in bold text.
(B–E) HCDR3s are colored by mAb according to the legend in (A).
See also Figure S3.