Figure 3.
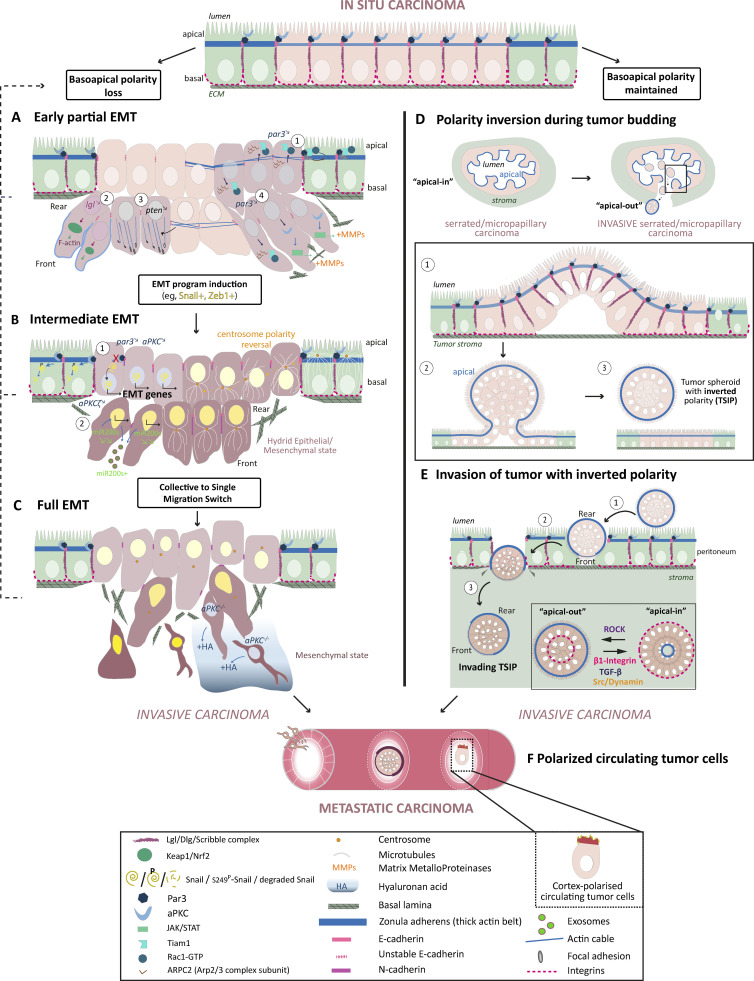
Basoapical polarity changes enhance cancer-invasive properties. Two main types of basoapical polarity changes turn in situ carcinomas into invasive and metastatic cancers: the progressive loss of basoapical polarity and the inversion of basoapical polarity. Progressive basoapical polarity loss is caused directly by cell polarity protein alterations or indirectly by disorganization of cell–cell and cell–ECM adhesions. Basoapical polarity loss is at the heart of the epithelial-to-mesenchymal transition. Progressive acquisition of mesenchymal traits turns stationary carcinoma cells into highly invasive cells (A–C). See Fig. 2 and text box for definitions of EMT. (A) Cell polarity protein alterations can trigger carcinoma invasion without fully activating EMT transcription programs. Because the complete absence of mesenchymal traits can’t be ruled out, this type of phenotypic changes could be at the very beginning of the EMT spectrum and is thus called early partial EMT. The cancer cells retain intercellular connections and epithelial traits but remodel their actin network to promote cell migration and weaken cell–cell adhesion (A1–A3). Molecular alterations include downregulation of Par3 expression leading to mislocalization of Rac1 activity and the Arp2/3 complex resulting in loosened actin belt at the zonula adherens (A1). Lgl and PTEN knockdown both promote collective cancer invasion by affecting the actin cytoskeleton (A2 and A3). Finally, par3 downregulation could also favor collective invasion by promoting MMPs’ secretion and ECM degradation, two traits usually associated with mesenchymal cells (A4). (B) Cell polarity protein alterations such as Par3 and aPKC downregulation can directly cause activation of EMT transcription by uplifting their inhibitory targeting of the EMT transcription factor Snail1 (B1). aPKCζ depletion indirectly triggers EMT program by reducing the intracellular level of miR200s via promotion of its secretion into exosomes, which activates Zeb1 expression, another EMT transcription factor (B2). Intermediate acquisition of the mesenchymal phenotype leads to an epithelial/mesenchymal hybrid state, which favors collective carcinoma invasion as cells retain strong enough intercellular adhesions. (C) Full epithelial-to-mesenchymal conversion and switch toward single cancer cell invasion is often observed in advanced metastatic cancer such as mesenchymal colorectal cancer. The absence of both aPKC kinases in intestinal stem cells is sufficient to trigger such cancers, whose invasive phenotype is promoted by the secretion of the ECM molecule hyaluronan acid (HA). (D) Carcinoma cells with intact basoapical polarity can collectively invade the tumor stroma after forming a bud which detach from the tumor core. This is typically observed in the micropapillary carcinoma and the serrated colorectal carcinoma. In the latter case, abnormal cancerous epithelium of intestinal glands bud apically within the lumen. As a result of this phenomenon, cancer spheroid bearing a supracellular actomyosin cortical belt on the outside detach and fill the lumen (D1–D3). The lumen of these glands eventually collapses which releases the spheroid in the stroma. (E) Once in the stroma, the spheroid contacts ECM molecules to repolarize their apical on the inside (“apical-in”). However, in some cases, this conversion does not occur leaving the tumor spheroid with an inverted basoapical polarity (TSIP). Interestingly these TSIP are able to invade the peritoneum, are highly metastatic and better resist chemotherapy than “apical-in” spheroids. The invasive process of the TSIP is just beginning to be understood (E1–E4). They likely perform a collective amoeboid mode of migration allowed by polarized supracellular actomyosin contractility at the rear of the spheroid coupled to the general jiggling of the cells, which generates friction forces necessary for forward movement (Pagès et al., 2022). (F) Finally, whereas clustering of individually invading mesenchymal carcinoma cells within the vasculature promotes metastasis success, single circulating tumor cells can increase their chance of secondary metastasis growth by polarizing their cortex to increase adhesion and extravasation.