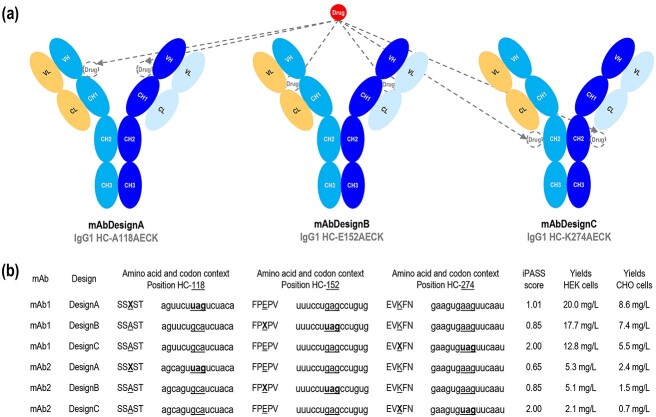
Figure 2.
Antibody synthesis. (a) Schematic representations of the mAb designs A, B, and C, with their intended drug location. (b) Amino acid context, codon context, theoretical iPASS score, and antibody yields in CHO and HEK systems for designs A, B, and C with mAb1 and mAb2. The reference amino acid or reference codon is indicated as underlined. X represents the non-canonical amino acid. iPASS scores were calculated based on the amber codon context by using the website shiny.bio.lmu.de:3838/iPASSv2. An iPASS score of ≥1 should indicate above-average relative non-canonical amino acid incorporation efficiency in HEK cells with the orthogonal M. mazei pyrrolysine-tRNA/pyrrolysine-RS pair. Antibody yields were quantified from the culture supernatants by affinity chromatography using the MabSelect™ SuRe™ column in an ÄKTA pure 25 L system. Transient transfections were independently performed three times. Amino acids are numbered according to IgG1-Eu described by Edelman et al. [19].