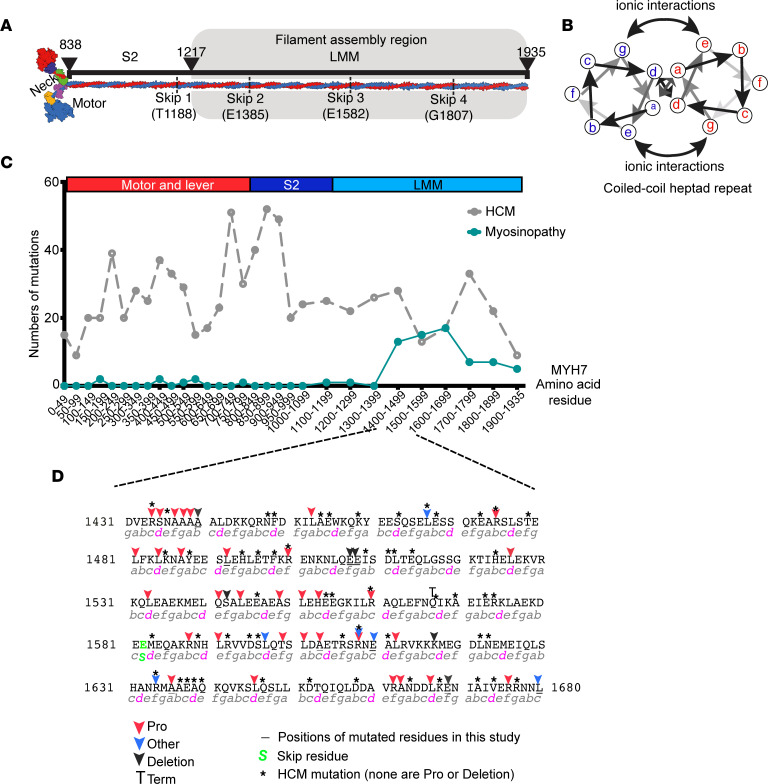
Figure 1. Myosinopathies and location of mutations.
(A) A schematic showing the overall composition of striated myosin. The molecule is formed by 2 heavy chains that dimerize to form a coiled coil tail composed of subfragment-2 (S-2) and light-meromyosin (LMM). The 2 heavy chains diverge to form the neck of lever, to which light chains bind, and the 2 motor domains, which bind actin and nucleotide. (B) A schematic showing an end on view of the heptad repeat of 2 interacting α-helices. Residues in “a” and “d” positions form the hydrophobic seam. (C) The frequency of mutations in MYH7 for hypertrophic cardiomyopathy (HCM) (gray dotted line) and for skeletal myopathies (myosinopathy, green line), across the amino acid sequence of MYH7. (D) The sequence in which mutations that cause myosinopathies are most frequent. Positions of mutations (commonly mutation to proline or a single amino acid deletion) are indicated by colored arrows. Mutations in residues mutated in HCM are indicated by an asterisk. Underlined residues indicate the position of mutated residues studied here. The heptad repeat is shown underneath the sequence, with “d” positions highlighted in magenta.