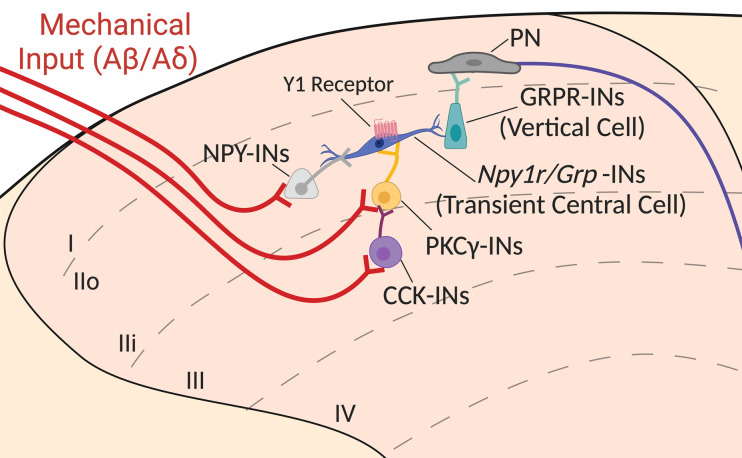
Figure 7. Schematized model for NPY Y1 agonists to inhibit Grp/Npy1r-INs and dampen neuropathic pain, silencing a key component of the ascending circuit in the dorsal horn that mediates mechanical allodynia.
In the context of nerve injury, aberrant hyperexcitation of Npy1r/Grp-INs may drive allodynia. Exogenous administration of NPY or Y1 agonist binding to the Gi-coupled NPY Y1 receptor on Npy1r/Grp-INs results in cellular inhibition and the abolishment of peripheral nerve injury–induced mechanical allodynia. We posit that NPY Y1 agonists act by inhibiting a key neuron population implicated in the transduction of mechanical allodynia. Briefly, non-noxious mechanical stimuli activate Aß/Aδ myelinated afferents (shown in red) that project into the deeper laminae of the dorsal horn and synapse onto interneurons marked by the expression of CCK (purple) and PKCγ (yellow). Normally, feed-forward inhibition prevents the activation of these interneurons, and as a result light touch is perceived as nonpainful. For example, inhibitory NPY interneurons (light gray) may “gate” Npy1r/Grp-INs to prevent these neurons from being activated and driving pain-like behaviors. However, in the context of neuropathic pain, feed-forward inhibition is lost, and innocuous light touch inputs activate a theorized dorsally directed microcircuit to allow innocuous mechanical sensory information to be perceived as painful. In this theorized circuit, activated CCK and PKCγ interneurons excite transient central cells (theorized here as Npy1r/Grp-INs), which in turn synapse onto GRPR-INs (vertical cells), which then activate ascending projection neurons (PNs) that travel via the spinothalamic and spinoparabrachial tracts to be processed via higher order pain centers, such as the lateral parabrachial nucleus. Image is updated from our circuit diagrams previously published under CC BY license (24, 26).