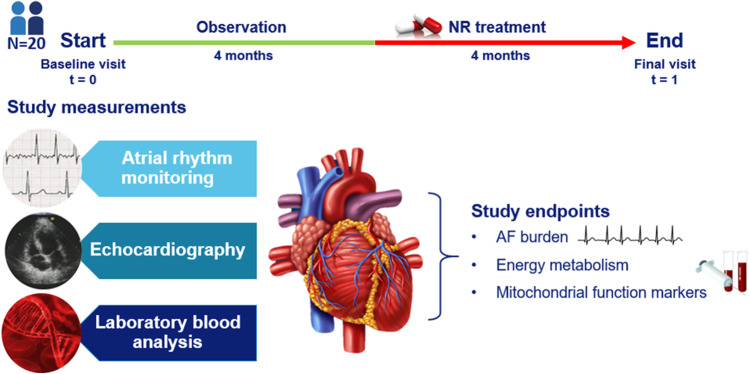
Fig. 1.
Clinical trial design. At baseline (t = 0), the average AF burden is calculated by remote rhythm monitoring using the atrial lead. Cardiac function is examined by echocardiography; energy metabolism and mitochondrial function marker are determined by laboratory blood analysis. After baseline, 4 months of NR supplementation follows. At follow-up (t = 1), the same clinical measurements are performed. Pre- and post-treatment outcomes are compared to determine the cardioprotective effects of NR on AF burden, mitochondrial function markers and energy metabolism