Figure 7:
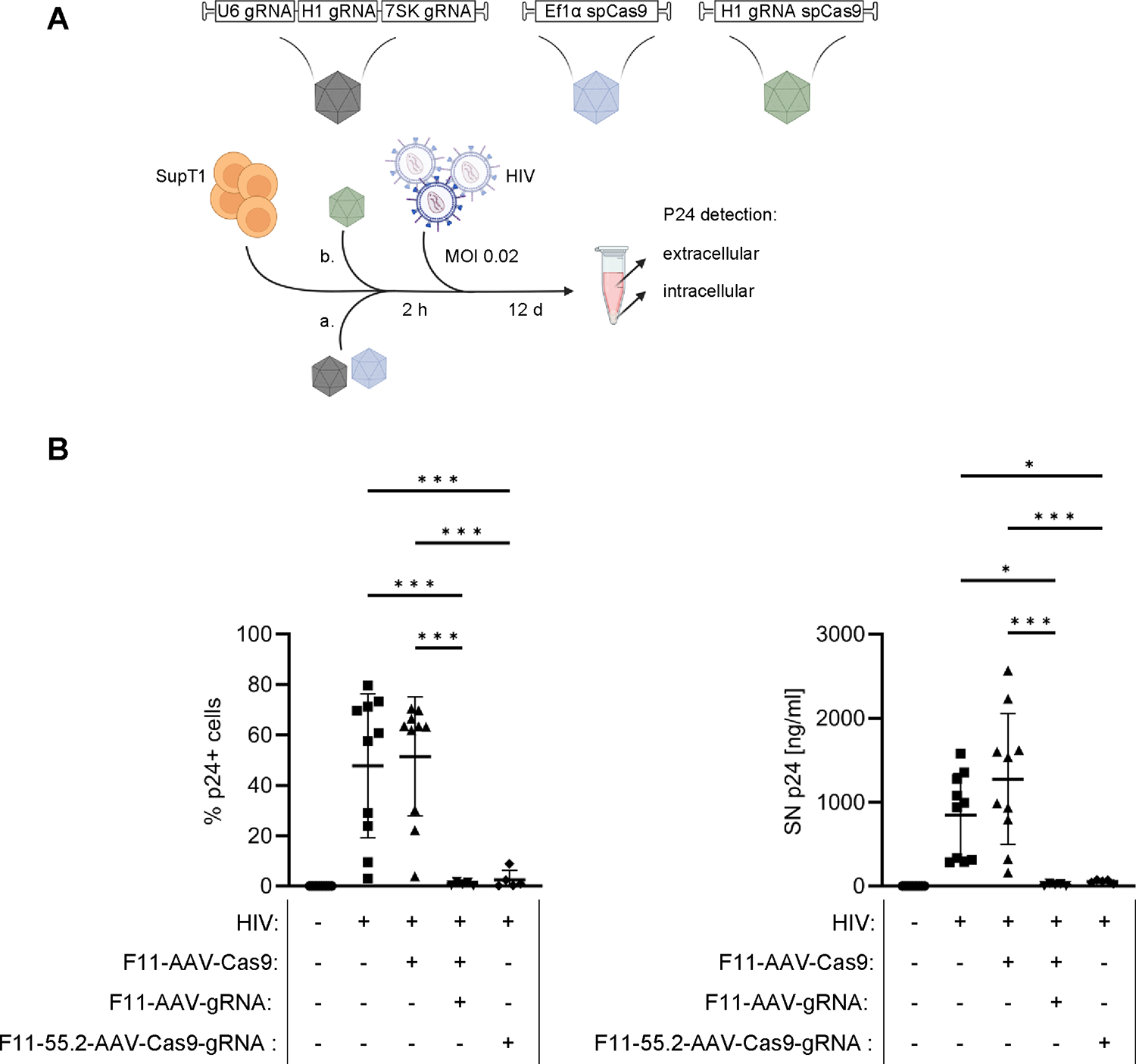
HIV-targeted endonuclease delivery by F11-AAV and F11-55.2-AAV.
A) Workflow for anti-HIV assay. SupT1-CD4/CD32a cells were incubated with F11-AAVs separately encoding Cas9 and gRNAs directed against two different sequences of the HIV-1 genome (a) or an all-in-one vector packaged in F11-55.2-AAV (green) (b). Two hours after transduction, cells were infected with HIV at an MOI of 0.02. Subsequently, CA-p24 protein concentration was analyzed in supernatant via ELISA and by intracellular staining at 12 days post infection. B) Percentages of CA-p24-positive cells (left) and CA-p24 levels in cell supernatants (right). Uninfected n=10, HIV infected n=10, HIV infected + F11-AAV-Cas9 n=10, HIV infected + F11-AAV-Cas9 + F11-AAV-gRNA n=5, HIV infected+ F11-55.2-AAV-Cas9-gRNA n=5. Statistical differences of technical replicates were calculated by one-way analysis of variance (ANOVA) followed by Tukey’s multiple comparisons test. SD = standard deviation. ***p = 0.0001–0.001, *p = 0.01–0.05. Only significant and relevant statistical comparisons are shown. The statistical comparison of all groups in each data set is available in the supplements.
