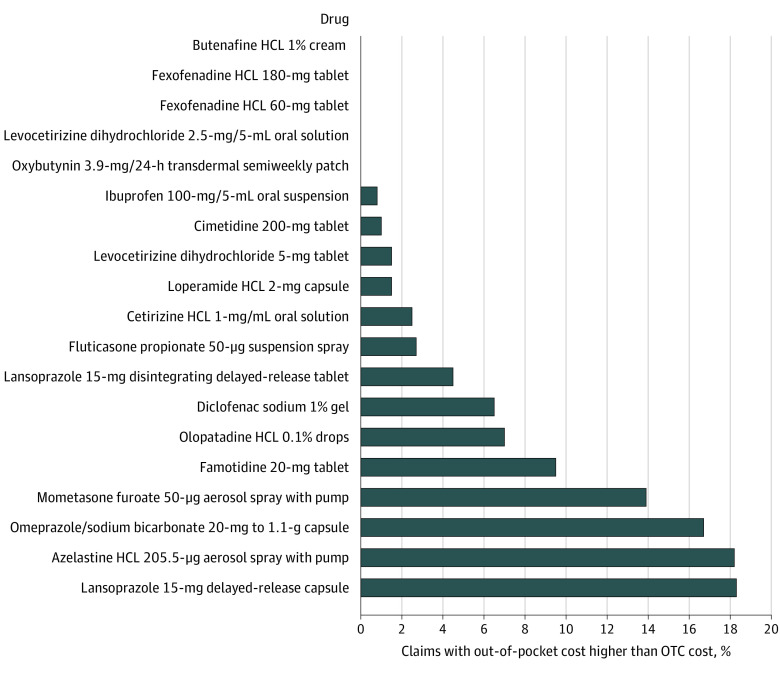
Figure. Postdeductible Claims for Which Medicare Part D Beneficiary Out-of-Pocket Costs Were Greater Than Over-the-Counter Prices.
The number of claims and beneficiary spending were obtained from Medicare Part D pharmacy claims data from a 20% national random sample of beneficiaries enrolled in traditional Medicare or Medicare Advantage. Beneficiaries who received a low-income subsidy or were eligible for Medicaid at any point during 2020 were excluded. Only claims that were filled after the deductible phase were included, to reflect claims where Medicare Part D coverage was applied. The included claims represented 82% of all claims for these drugs among included beneficiaries. Over-the-counter (OTC) cash prices were obtained from the Amazon price tracker site Keepa (keepa.com) for December 31, 2020, except for azelastine and mometasone furoate, whose earliest available prices were from August 2022. If multiple vendors were available for the same drug, the product with the highest number of reviews was chosen. The OTC cash price did not include shipping costs or sales taxes. Cash prices for OTC products were transformed to a per-unit basis using the same units recorded in the Medicare claims. Medicare beneficiary out-of-pocket cost per unit reflects total patient out-of-pocket cost divided by the total number of units dispensed for the drug in Medicare Part D in 2020. The data do not include 2 drugs (fexofenadine hydrochloride [HCL] 30-mg tablets and ranitidine HCL 150-mg tablet) that did not have claims in Medicare Part D in 2020 and 1 drug (omeprazole/sodium bicarbonate 20- to 1680-mg packet) for which historic prices were not available.