Abstract
Glioma is the most prevalent type of brain tumor characterized by a poor 5-year survival rate and a high mortality rate. Malignant gliomas are commonly treated by surgery, chemotherapy and radiotherapy. However, due to toxicity and resistance to chemoradiotherapy, these treatments can be ineffective. Anxiety and depression are highly prevalent in patients with glioma, adversely affecting disease prognosis and posing societal concerns. Ferroptosis is a type of non-apoptotic, iron-dependent cell death characterized by the accumulation of lethal reactive oxygen species produced by iron metabolism, and it serves a key role in numerous diseases. Regulation of iron phagocytosis may serve as a therapeutic strategy for the development of novel glioma treatments. The present review discusses the mechanisms underlying the occurrence and regulation of ferroptosis, its role in the genesis and evolution of gliomas, and its association with glioma-related anxiety and depression. By exploring potential targets for glioma treatment, the present review provides a theoretical basis for the development of novel therapeutic strategies against glioma.
Keywords: glioma, ferroptosis, anxiety, depression, pathway, metabolism
1. Introduction
Global statistics show that ~49% of malignant brain tumors are glioblastomas and 30% are diffuse infiltrating low-grade gliomas (1). The 2021 World Health Organization (WHO) classification central nervous system (CNS)5 is a new system that combines the histological features and molecular phenotypes of tumors. According to this classification system, gliomas can be divided into adult diffuse gliomas, diffuse low-grade gliomas in children, diffuse high-grade gliomas in children and localized astrocyte gliomas. Furthermore, gliomas are divided into four grades based on the degree of malignancy: WHO Grades I and II are classified as low-grade gliomas, whereas WHO Grades III and IV are classified as high-grade gliomas (2). High-grade gliomas, such as glioblastoma, are associated with poor outcomes and a 5-year relative survival rate of <6.9% (3). Thus, early identification and treatment are crucial for improving the prognosis of patients with gliomas (4).
Traditional glioma treatment typically involves surgery, chemotherapy and radiation. The biocomplexity of glioma cells, including their heterogeneity, high proliferation rate and infiltration, contributes to their high recurrence and drug resistance. Despite continual improvement in the understanding of the pathological mechanism of glioma, the quality of life of patients remains dismal (5). Thus, identifying the most effective targets and treatments for glioma is essential.
In addition to the challenges of disease progression and recurrence, psychological problems, such as anxiety and depression, often characterize patients with gliomas (6). Individuals with anxiety disorders frequently exhibit symptoms of depression, and those with depression frequently experience anxiety disorders. The two conditions can occur concurrently, and individuals can meet the criteria for both. These two widely prevalent psychiatric disorders substantially contribute to morbidity and mortality worldwide. Comorbid depression and anxiety disorders occur in up to 25% of general practice patients in Australia (7). Comorbid anxiety and depression are particularly attributed to shared predisposition or the possibility that one condition can manifest as an epiphenomenon of the other (8). A previous study reported the association of neuroinflammation, oxidative stress and nitrosative stress with depression and its comorbidities (9). Moderate to severe depression and anxiety were diagnosed in 28 and 36% of patients with glioma, respectively (10). Although their precise causes remain unknown, anxiety and depression remain severe problems in patients with glioma (11). Furthermore, a retrospective study illustrated that patients with glioma and depression are associated with shorter overall survival (OS) when compared with patients without depression, with median survival times of 7 and 11 months, respectively. Preoperative depression has been identified as an independent factor predicting poorer OS in patients with glioma.
Ferroptosis is a novel form of iron-dependent programmed cell death characterized by specific hallmarks, such as glutathione depletion, decreased glutathione peroxidase activity and the inability of lipid oxides to be processed through the glutathione peroxidase (GPX) pathway, which is catalyzed by GPX4. Ferroptosis is promoted by the oxidation of lipids by divalent iron ions, resulting in the generation of reactive oxygen species (ROS) (12). Recent studies have reported that ferroptosis is a significant factor in certain malignancies and degenerative disorders, including gliomas (13), triple-negative breast cancer (14), colorectal cancer (15), liver cancer (16) and kidney cancer (17). Regulating ferroptosis is a potential therapeutic approach towards the development of new treatment modalities.
2. Iron metabolism
Iron is indispensable to life, having a crucial role in numerous physiological activities. Specifically, iron-containing enzymes are involved in key physiological activities, such as ATP production, DNA synthesis and oxygen transport (18). Furthermore, iron serves an essential role in brain development and function, and is involved in various biological processes, such as embryonic neuronal development, myelination, neurotransmitter synthesis and oxidative phosphorylation (19). Iron is also vital for the activity of enzymes involved in the production of monoamines (such as dopamine, epinephrine, norepinephrine and serotonin), which are associated with social-emotional development, executive functioning and memory processes. However, iron deficiency compromises the activities of iron-dependent enzymes in all tissues. Furthermore, although iron promotes cell division and growth, it can also lead to cell damage, as excessive iron accumulation can be toxic, activating cell death signaling pathways through oxidative stress (20).
Previous studies have demonstrated a relationship between iron metabolism dysregulation and certain illnesses, including cancer, neurological diseases and atherosclerosis (21,22). To maintain adequate and safe iron levels, cells express diverse coordinated proteins that strictly regulate both intracellular and systemic iron metabolism. Among these proteins, iron transporters play a crucial role in controlling iron absorption, storage, distribution and overall iron homeostasis (23).
Iron absorption
The apical brush border membranes of the small intestine absorb both heme and non-heme iron. To facilitate this process, ferric reductase duodenal cytochrome b converts non-heme Fe3+ entering the colon cells to ferrous ions (Fe2+). Divalent metal transporter 1 (DMT1), a proton-coupled transporter found on the apical membrane of intestinal epithelial cells, absorbs dietary non-heme iron (mainly ferric, Fe3+) (24). Iron-chaperone poly(C)-binding protein 2 (PCBP2) mediates the transfer of iron to ferritin (25). PCBP2 binds to DMT1 and ferroportin (FPN), encoded by the solute carrier family (SLC)40 member A1 gene] to facilitate the export of iron from the epithelial cell layer into the bloodstream (Fig. 1A) (24,26–30).
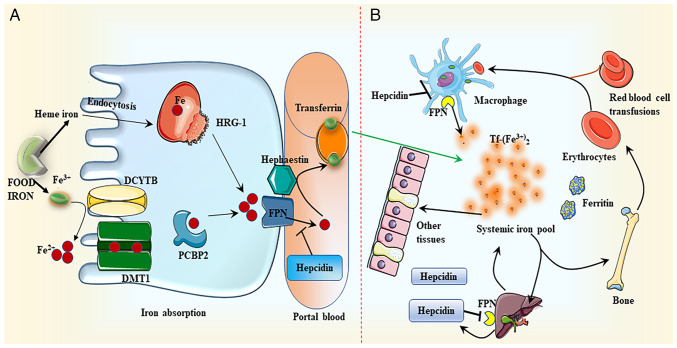
Figure 1.
Iron metabolism. (A) Iron absorption. DCYTB mediates the conversion of dietary non-heme iron (Fe3+) to ferrous ion (Fe2+), which is then absorbed by DMT1 in the membrane of intestinal epithelial cells. Following this intake, iron is either retained in ferritin or transferred to the basement membrane by the iron-chaperone PCBP2, where it is subsequently converted to Fe3+ by hephaestin. Finally, iron is discharged to the portal circulation through FPN. Tf attaches to the exported iron, which is then transported to numerous peripheral tissues. HRG-1 may uptake heme through endocytosis. Heme is degraded by heme oxygenase once it has been absorbed. Similar to the transport of non-heme iron, iron liberated from heme is transferred to portal vein blood through FPN. (B) Iron distribution. Hepcidin regulates the production of FPN by directly binding to FPN and promoting its breakdown, thus facilitating the transport of iron to the portal vein. Iron is mainly distributed in red blood cells, which transport oxygen in the blood, and tissues, such as muscle, liver and bone marrow. DCYTB, duodenal cytochrome b; DMT1, divalent metal transporter 1; PCBP2, poly(C)-binding protein 2; FPN, ferroportin; HRG-1, heme responsive gene-1; Tf, transferrin.
Although the mechanisms of heme iron absorption are not entirely clear, two types of carrier proteins are hypothesized to be involved. Firstly, heme carrier protein 1 (HCP1) has initially been associated with iron absorption. However, HCP1 has been identified to exhibit high affinity for folate, and primarily functions as a folate transporter instead of an iron transporter (31). Secondly, the role of heme-responsive gene 1 (HRG-1) has gained attention. HRG-1 exhibits a high sensitivity to heme and may activate the endocytosis pathway for heme trafficking into the cytosol (Fig. 1) (32). Next, heme is broken down by heme oxygenase, producing Fe2+, which is then metabolized in the same pathway as non-heme iron (33).
Iron transport
Cells primarily absorb plasma transferrin (Tf)-bound iron through Tf receptor (R)1. The interaction between Fe2+ and TfR1 at the plasma membrane induces receptor-mediated endocytosis of the Tf/TfR1 complex. In the nucleus, DMT1 transfers iron from the Tf/TfR1 complex to the cytoplasm after reduction by the prostate epithelial transmembrane protein, converting it to Fe2+. Tf/TfR1 is then recycled to the cell surface and released into the plasma. After transportation to the peripheral blood, hephaestin converts Fe2+ to Fe3+. Iron is chelated and redox-active in the labile iron pool (LIP) within the cell. Iron from the LIP is transported to different cell regions to meet metabolic demands or stored in ferritin (Fig. 1) (34).
The blood-brain barrier (BBB) and blood-cerebrospinal fluid (CSF) barrier (BCSFB) separate the central nervous system from the dynamic environment of the bloodstream (35). The BBB is a complicated multicellular system composed of endothelial cells and a distinct basement membrane (36–38). The BCSFB is located in the choroid plexus of the lateral, third and fourth cerebral ventricles. A carrier system of choroid plexus epithelial cells can facilitate material exchange between CSF and blood. Thus, the transportation of iron across the BBB and BCSFB is necessary for iron to enter the brain (39,40).
The Tf/TfR1 pathway is a potential route through which iron transporters can cross the luminal (apical) membrane of the BBB. Hephaestin (HEPH) and FPN1/ceruloplasmin (CP) mediate the transport of iron through the luminal membrane (41,42). The Tf/TfR1/DMT1 pathway is a key iron transport mechanism through the BCSFB. Moreover, the FPN1/CP or FPN1/HEPH pathway mediates iron export from the choroidal epithelium to the CSF. The degree of TfR1 expression in neurons is proportional to their iron needs. Neurons express DMT1, which transfers iron from Tf to the cytoplasm (43). Furthermore, DMT1 is implicated in the iron uptake of hippocampal neurons during maturation and memory formation (44,45).
Astrocytes in the central nervous system engage in intracellular iron transport to maintain extracellular iron balance. DMT1 may mediate this uptake, and FPN1 and CP have been demonstrated to be highly expressed on astrocyte membranes; these two proteins may be necessary for iron to leave astrocytes and enter the extracellular brain region (46,47).
3. Lipid metabolism
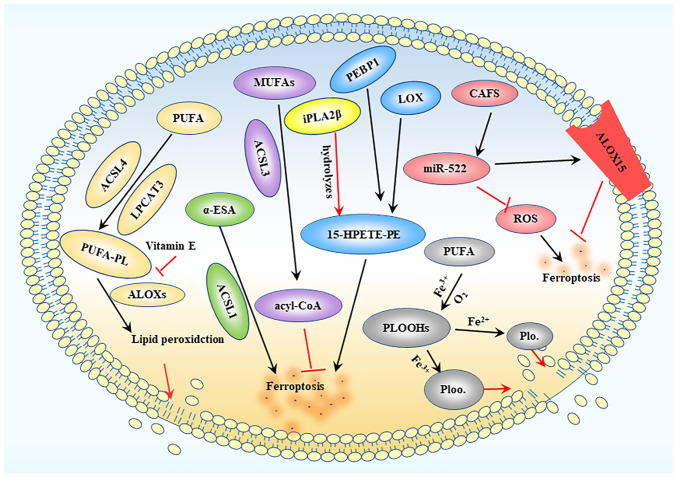
Lipid peroxidation serves as a hallmark of ferroptosis, a process that directly damages cell membranes and leads to ferroptotic cell death. This buildup of lipid peroxides in cell membranes is initiated by the inhibition of glutathione (GSH) production, import of cysteine from the extracellular environment and direct inhibition of GPX4 activity. Lipid peroxidation predominantly occurs via enzymatic and non-enzymatic oxidation (48). The lipoxygenase (LOX) family, which oxidizes free and esterified polyunsaturated fatty acids (PUFAs) to generate peroxide radicals, controls enzymatic lipid peroxidation. Acyl-CoA synthetase long chain (ACSL) family member 4 and lysophosphatidylcholine acyltransferase 3 facilitate the binding of PUFAs to phospholipids, thereby producing PUFA phospholipids (PUFA-PLs). PUFA-PLs are susceptible to lipid peroxidation induced by arachidonic acid (A)LOXs, which eventually results in the loss of the lipid bilayer and impairs cell membrane function, thus increasing ferroptosis (49).
Radiotherapy can increase the expression of ACSL4, thereby enhancing ferroptosis in glioma cells. Meanwhile, ACSL1 is responsible for ferroptosis induced by conjugated linoleic acid. By contrast, ACSL3 transforms monounsaturated fatty acids into acyl-CoA esters, which bind to membrane phospholipids, thereby preventing ferroptosis in cancer cells. Moreover, cancer-associated fibroblasts secrete microRNA-522 through the exosome pathway, which inhibits cancer cell ferroptosis by targeting ALOX15 and inhibiting the accumulation of lipid-ROS. Additionally, vitamin E can suppress ALOX activity (50). However, although LOX activators alone do not directly cause ferroptosis, a previous study reported that ALOXs overexpression enhanced sensitivity to the small compound erastin. It has been suggested that overexpression of wild-type ALOX15, rather than ALOX15 with an N-terminal truncation, enhances iron deposition induced by Erastin (51). In addition, LOX is crucial for ferroptosis when GSH is depleted (49). In most cases, LOX may not be the primary driver of ferroptosis, but it may contribute to the initiation or spread of damage. The 15-LOX/phosphatidylethanolamine-binding protein 1 (PEBP1) complex is produced through the interaction of LOX with PEBP1. Ferroptosis is initiated by the ferroptosis signal molecule 15-hydroperoxy-eicosa-tetraenoyl-phosphatidylethanolamine (49) (Fig. 2).
Figure 2.
Lipid peroxidation process. ACSL4 and LPCAT3 mediate PUFA binding to phospholipids to produce PUFA-PLs, while ALOXs further induce the production of lipid peroxides, ultimately destroying the lipid bilayer. MUFA is converted into acyl-CoA under the action of ACSL3, thereby inhibiting ferroptosis. LOX interacts with PEBP1 to produce 15-HPETE-PE, leading to ferroptotic death. CAFs produce extracellular vesicles containing miR-522, which can inhibit ROS accumulation and target ALOX15 to inhibit ferroptosis. ACSL, acyl-CoA synthetase long chain; LPCAT3, lysophosphatidylcholine acyltransferase 3; PUFA, polyunsaturated fatty acid; PUFA-PL, PUFA phospholipid; MUFA, monounsaturated fatty acid; LOX, lipoxygenase; ALOX, arachidonic acid LOX; PEBP1, phosphatidylethanolamine-binding protein 1; 15-HPETE-PE, 15-hydroperoxy-eicosa-tetraenoyl-phosphatidylethanolamine; CAFs, cancer-associated fibroblasts; miR, microRNA; ROS, reactive oxygen species; PLOOH, phospholipid hydroperoxide; iPLA2β, calcium-independent PLA2β; α-ESA, α-eleostearic a.
4. Ferroptosis
System xc−
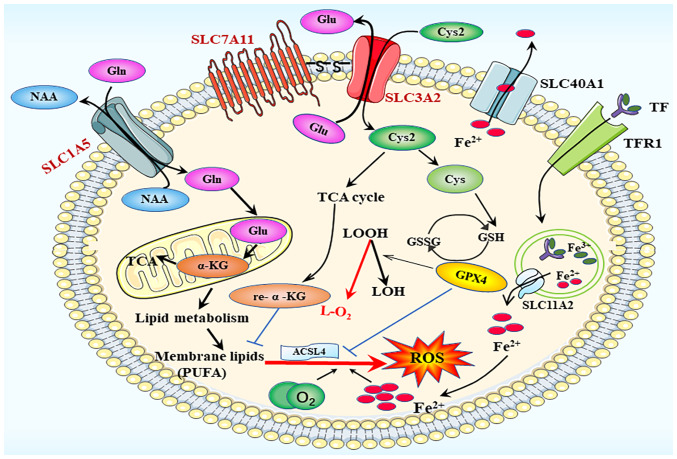
Cysteine deficiency serves a significant role in the induction of ferroptosis and is a major contributor to ferroptosis in glioblastoma (52). In the cell membrane, the amino acid transport system xc− is composed of two key components, SLC7A11 (xCT) and SLC3A2 (also known as 4F2 cell-surface antigen heavy chain). This system facilitates the exchange of glutamate and cysteine. Once inside the cell, cysteine 2 (Cys2) is converted into cysteine, which stimulates the production of the GPX4 substrate GSH (53). GPX4, a fundamental regulator of ferroptosis, may convert GSH to oxidized glutathione and reduce lipid hydroperoxides to lipid alcohols. This mechanism is essential for the prevention of lipid peroxidation and inhibition of ferroptosis (54). Both GPX4 knockdown and inactivation result in ferroptosis (55). By blocking cysteine transport through system xc−, the ferroptosis inducer elastin can cause GSH depletion and GPX4 inactivation (56), whereas RAS-selective lethal 3 directly promotes ferroptosis by reducing GPX4 activity. Cysteine is an essential limiting amino acid for the synthesis of intracellular GSH, and GPX4 function is immediately affected by GSH depletion. Thus, system xc− that is responsible for Cys2 absorption is considered to be one of the most important regulators of ferroptosis (Fig. 3).
Figure 3.
Ferroptosis process. The main participating system in ferroptotic death is the amino acid transport system xc−, composed of the SLC1A5 and SLC3A2 families. SLC, solute carrier family; ACSL4, acyl-CoA synthetase long chain family member 4; GPX4, glutathione peroxidase; GSH, glutathione; GSSG, glutathione disulfide; ROS, reactive oxygen species; TfR1, transferrin receptor 1; α-KG, α-ketoglutarate; Cys, cysteine; Cys2, cysteine2; Glu, glutamate; Gln, glutamine; LOH, lipid alcohol; LOOH, lipid hydroperoxidecid; TCA cycle, tricarboxylic acid cycle.
SLC1A5 and SLC3A2
SLC1A5 and SLC3A2 are essential proteins for the transmembrane translocation of glutamine into cells (57). Once inside the cells, glutaminases are transported to the mitochondria and glutamine is converted into glutamate and ammonia. Glutamate can then be transformed into α-ketoglutarate (α-KG), a crucial step in the tricarboxylic acid cycle (Fig. 3) (58). Dihydrolipoamide dehydrogenase (DLD) serves a crucial role in the promotion of ferroptosis, especially in cases of cysteine deficiency or inhibition of cysteine import. α-KG can stimulate DLD to generate hydrogen peroxide, and it can also be converted into acetyl-CoA, thereby enhancing fatty acid production and promoting lipid peroxidation-dependent ferroptosis (59).
Gene transcriptional regulatory network
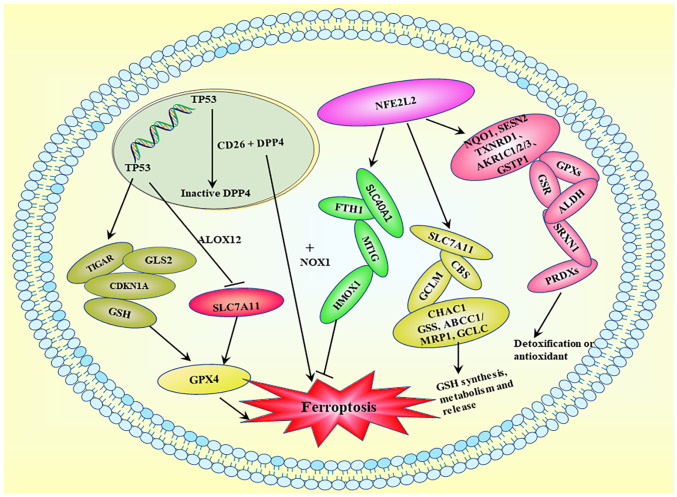
Complex transcriptional regulatory networks affect the cell vulnerability to ferroptosis. Several transcription factors have been demonstrated to control certain ferroptosis-related genes (60). For instance, the transcription factors tumor protein p53 (TP53), activating transcription factor (ATF)3, BTB domain and CNC homolog 1 (BACH1) and STAT1 upregulate SLC7A11 and downregulate nuclear factor erythroid 2-related factor 2 (NFE2L2/Nrf2), ATF4 and aryl hydrocarbon receptor nuclear translocator-like protein 1. The intricate roles served by various transcription factors associated with ferroptosis, including TP53 and NFE2L2, are shown in Fig. 4. TP53 upregulation has been shown to suppress expression of the system x−c transporter subunit SLC7A11 and sensitize cells to ferroptosis (18).
Figure 4.
Ferroptosis-related transcriptional regulation. Activation of TP53 exerts a minimal effect on GSH levels or GPX4 function, but it downregulates SLC7A11 and inhibits cysteine absorption. Activation of TP53 results in the production of GSH, CDKN1A, GLS2 and TIGAR. DPP4/CD26 interacts with TP53 in the nucleus and is maintained in a dormant state, whereas TP53 deficiency promotes DDP4 cell membrane localization. DDP4 induces ferroptosis by binding to NOX1 on the cell membrane. TP53, tumor protein p53; GSH, glutathione; GPX, glutathione peroxidase; SLC, solute carrier family; CDKN1A, cyclin-dependent kinase inhibitor 1A; GLS2, glutaminase 2; TIGAR, TP53-induced glycolytic regulatory phosphatase; DPP4, dipeptidyl peptidase 4; NOX, NADPH oxidase; NFE2L2, nuclear factor erythroid 2-related factor 2; ALOX, arachidonic acid lipoxygenase; FTH1, ferritin heavy chain.
NFE2L2 exerts multiple effects on ferroptosis via transcriptional regulation. First, it inhibits ferroptosis by upregulating iron metabolism genes, including FTH1, SLC40A1, HMOX1 and MT1G. Second, NFE2L2 activation enhances the expression of SLC7A11, GCLM, CBS, CHAC1, ABCC1/MRP1, GCLC and GSS, which are involved in GSH production, metabolism and release. Third, several NFE2L2 target genes, including NQO1, TXNRD1, AKR1C1/2/3, SESN2, GSTP1, GPXs, GSR, SRXN1, ALDH and PRDXs, participate in detoxification or antioxidant activities, which may reduce ferroptosis sensitivity. Therefore, NFE2L2 serves a crucial role in the transcriptional regulation of ferroptosis, primarily via the activation of genes that counteract cellular damage (61).
5. Regulation of iron homeostasis
The maintenance of iron homeostasis is crucial. Hepcidin, a 25-amino acid peptide hormone synthesized and released by the liver, serves a key role in the regulation of iron storage, distribution and consumption. It regulates FPN production by directly binding to FPN and promoting its breakdown (62). An excessive increase in iron levels induces the production of hepcidin, which destroys FPN in intestinal epithelial cells, thus reducing plasma iron levels. By contrast, under iron-deficient conditions, hepcidin levels decrease to maintain FPN expression, facilitating the release of iron into the plasma (63). As aforementioned, DMT1 and TfR1 are essential proteins found in cells that help regulate intracellular iron concentration, thereby serving a vital role in iron homeostasis. Elevated iron levels can lead to the formation of hydroxyl radicals through the Fenton reaction, particularly when combined with hydrogen peroxide. This process induces the oxidation of PUFAs in cell membranes, substantially accelerating lipid peroxidation and ultimately causing cell damage or death (64).
In the brain, the BBB and BCSFB serve crucial roles in maintaining the stability of physical and chemical elements in the brain tissue environment. The BBB is the most significant barrier in the brain that prevents ~98% of small molecule reagents from passing when treating CNS-related disease (65). Moreover, these barriers regulate the transport of iron from the bloodstream to the brain parenchyma, helping to maintain brain iron levels largely independent of systemic iron levels and providing protection against systemic iron toxicity (37,38). In addition, iron ion equilibrium is maintained by three antioxidant mechanisms, namely GSH, selenium and Coenzyme Q (CoQ) systems.
GSH system
GSH is an antioxidant tripeptide composed of glutamic acid, cysteine and glycine. The enzyme glutamate cysteine ligase continuously catalyzes the production of GSH from glutamic acid and cysteine. However, the limited availability of cysteine within cells can decrease GSH production. To counteract this limitation, system xc− serves as a transport mechanism during GSH production. Inhibition of systemic xc-depletes intracellular cysteine, leads to a decrease in glutathione concentration and triggers oxidative stress, and increases the sensitivity of cells to ferroptosis (34). GSH serves a vital role in the system xc−/GSH/GPX4-dependent antioxidant defense pathway during ferroptosis, and it is essential for the maintenance of normal GPX4 activity (66,67). GPX4 has three subtypes: Mitochondrial, cellular solute and nuclear; it remains unclear which of these subtypes is mainly involved in the regulation of anti-ferroptotic effects (18).
Selenium system
Selenium, an essential trace element, regulates cellular redox processes during oxidative stress. In selenocysteine-containing proteins, such as GPX4, selenium serves a crucial role in the enhancement of their antioxidant function during ferroptosis. Selenium deficiency results in cellular death that depends on lipid peroxidation (68). A previous study reported that sodium selenite can reduce heme-driven ferroptosis triggered by homocysteine (a metabolite of methionine) or intracerebral hemorrhage. and enhanced the transcriptional upregulation of selenoprotein genes (including GPX4, Selenop and Txnrd1) and GPX3. However, it remains unclear whether selenoproteins other than GPX4 are also involved in the control of ferroptosis (69).
CoQ system
CoQ, commonly known as ubiquinone, is an endogenously generated and ubiquitous oxy-benzoquinone molecule. By promoting the movement of electrons from complexes I and II to complex III, CoQ10 substantially contributes to the electron transport chain. In addition, the lipophilic antioxidant CoQ can be transformed into panthenol, a reduced form that is considered to be involved in the recovery of other antioxidants (such as ascorbate and tocopherol), preventing cells from undergoing ferroptosis without the need for GSH (70). Apoptosis-inducing factor mitochondria-associated 2/ferroptosis-suppressor protein 1 (FSP1) regulates ferroptosis by synthesizing CoQH2 (Fig. 5) (71).
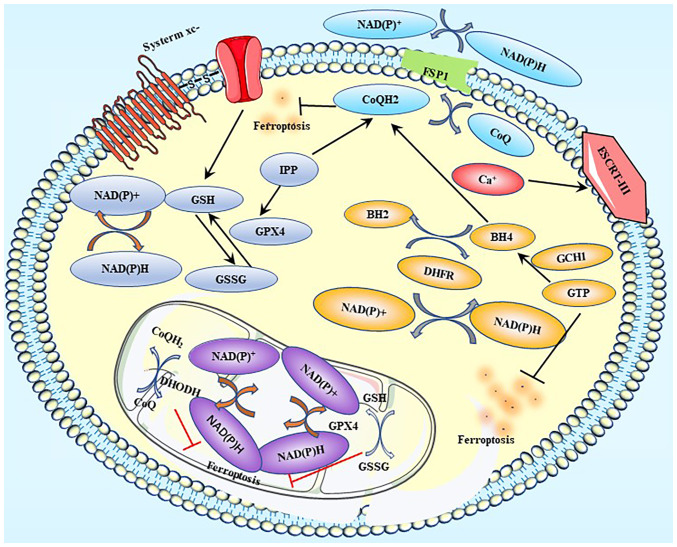
Figure 5.
Ferroptosis-related defense systems. Label A represents the GPX4-GSH axis; label B represents the FSP1-CoQ10-NAD(P)H pathway; label C represents the DHODH-mediated ferroptosis defense; label D represents the GCH1-BH4-DHFR axis; and label E represents the ESCRT III-mediated plasma membrane repair system. GPX, glutathione peroxidase; GSH, glutathione; GSSG, oxidized glutathione; FSP1, ferroptosis suppressor protein 1; CoQ, coenzyme Q; CoQH2, Coenzyme QH2; DHODH, dihydroorotate dehydrogenase; GCH1, GTP cyclohydrolase 1; BH4, tetrahydrobiopterin; BH2, dihydrobiopterin; DHFR, dihydrofolate reductase; ESCRT, endosomal sorting complex required for transport; IPP, Isopentenyl pyrophosphate.
There are at least five defensive mechanisms, including the GPX4-GSH axis, FSP1-CoQ10-NAD(P)H pathway, DHODH-mediated ferroptosis defense, GCH1-BH4-DHFR axis, and ESCRT III-mediated plasma membrane repair system, which are involved in the reduction or alleviation of the damage caused by ferroptosis (49) (Fig. 5).
6. Glioma
Ferroptosis, a form of programmed cell death driven by iron-dependent lipid peroxidation, has an unclear role in the death of glioma cells. In addition to depleting GSH and cysteine, phenylarsine oxide (PAB) has been reported to cause abnormal elevations in the levels of intracellular ferrous ion, H2O2 and lipid peroxides, reducing the survival of glioma cells both in vitro and in vivo. Deferoxamine, an iron chelator, has been reported to reduce PAB-induced lipid peroxidation and glioma cell death in vitro, whereas ferric ammonium citrate could reverse these effects. Ferrostatin-1- and GSH-induced suppression of lipid peroxidation inhibited glioblastoma (U87 and U251) cell death induced by PAB. Treatment with PAB resulted in intact cell membranes, fewer mitochondria, denser mitochondrial membranes, normal nuclear size and no chromatin condensation. PAB increased intracellular iron levels by activating Tf receptors. Excessive iron supply triggered the activation of NADPH oxidase 4 (NOX4), leading to excessive H2O2 and lipid peroxide production. Additionally, PAB increased the production of H2O2 and lipid peroxides by causing intracellular GSH depletion via the p53-mediated xCT pathway. Thus, PAB may induce glioblastoma multiforme cell ferroptosis, potentially serving as a glioma treatment (72).
Overexpression of the nuclear factor (erythroid-derived)-like 2 (NFE2L2) or suppression of the Kelch-like ECH-associated protein 1 (Keap1) has been shown to promote glioblastoma invasiveness and hence accelerate glioma growth (72). The Keap1/NFE2L2 signaling pathway can modulate ferroptosis activation (72). High mobility group box 1 protein has been reported to induce ferroptosis in mesangial cells by stimulating the Keap1/NFE2L2 signaling pathway (73). By downregulating NFE2L2 and inhibiting the nuclear receptor subfamily 2 group F member 6/KEAP1 signaling pathway, apatinib has been demonstrated to induce ferroptosis in glioma cells. Ibuprofen stimulates ferroptosis by inhibiting Keap1/NFE2L2 signaling. Moreover, NFE2L2 overexpression or Keap1 knockdown has been reported to expedite the proliferation and oncogenic transformation of glioblastoma U87 cells (74). Thus, the Keap1/NFE2L2 pathway may inhibit ferroptosis (75,76).
Similar to NFE2L2, xCT is positively regulated by ATF4 and serves a pivotal role in reducing ferroptosis-induced ROS production in glioblastoma multiforme cells. xCT can be induced by activating ATF4. Continuous targeting to promote ATF4-dependent processes activates xCT and thus enhances the growth of malignant gliomas (72). In addition, ATF4 has been reported to promote tumor angiogenesis in gliomas and affect the vascular architecture in an xCT-dependent manner. In a manner that is dependent on xCT, pseudolaric acid B also causes ferroptosis (77).
Ubiquitin thioesterase OTUB1 is abundantly expressed in gliomas. A novel axis of OTUB1/SLC7A11 contributing to the stemness of glioblastoma U373, U87 and U251 cell lines has been reported in a recent study (78). OTUB1 was demonstrated to stabilize SLC7A11 by directly interacting with it, while in the absence of OTUB1, ferroptosis was triggered by SLC7A11 expression.
In addition to limiting proliferation via vitamin C deficiency and ACSL4 inhibition, ferroptosis can be achieved by activating the transcription factors BACH1 or NOX4 to increase oxidative stress, restrict autophagy and trigger cell death. Drugs, such as 2-nitroimidazoles, temozolomide and artemisinin (and its derivatives) have been developed based on these principles. Downregulation of GPX4 and subsequent accumulation of lipid ROS are the key mechanisms through which dihydroartemisinin triggers ferroptosis. Ferrostatin-1, a specific inhibitor of ferroptosis, was reported to reverse all these changes (79). Additionally, temozolomide has been reported to induce ferroptosis by increasing DMT1 expression in the TJ905 glioblastoma cell line. Thus, DMT1 may be used as a therapeutic target for glioblastoma (80–82).
A study examining expression data from The Cancer Genome Atlas reported that a higher tumor grade and poor prognosis in patients with glioma were associated with the upregulation of coatomer subunit ζ-1 (COPZ1). Moreover, ferritin phagocytosis, which has been linked with the development of cancer and degenerative diseases, has been associated with nuclear receptor coactivator 4 (NCOA4) expression (83). Knockdown of COPZ1 can activate NCOA4, leading to the degradation of ferritin. The Fenton reaction, which is triggered by large concentrations of divalent iron, increases ROS production, and lipid peroxidation caused by ROS eventually leads to ferroptosis. Studies have indicated that depletion of COPZ1 induces ferroptosis in glioma cells by increasing NCOA4 and ATG7 levels. Thus, the COPZ1/NCOA4/FTH1 axis may be a novel therapeutic target in the treatment of glioma (84).
7. Anxiety and depression
The development of psychological disorders is a complex process that often involves the accumulation of multiple emotional changes instead of a single emotional shift. An increase in the prevalence of anxiety and depression is typically accompanied by an increase in suicide rates, which is a major concern in contemporary society. Depression and anxiety, the two most prevalent psychological diseases, are responsible for the morbidity and mortality of millions of individuals worldwide (85). Numerous biological, psychological and social environmental variables (86–88) contribute to the pathophysiology of depression and anxiety, such as personal experiences, workplace culture and significant life events. Psychological problems can exert diverse effects on physical health. Depression and anxiety disorders have been reported to co-occur with cardiovascular disease, trauma and numerous forms of cancer, such as lung cancer, breast cancer and malignant brain tumors (89). Cancer-related chronic pain and its management may lead to mental disorders, and depression and anxiety in particular can alter the synthesis of inflammatory cytokines and chemokines, the metabolism of neurotransmitters and the function of the neuroendocrine system (90).
Glioma is a fatal neurological disease associated with sexual dysfunction, cognitive impairment and possibly death. It is the most widespread and hazardous form of central nervous system tumor, account for 40–60% of all primary central nervous system tumors (91,92). Glioma is often accompanied by psychological disorders, such as anxiety and depression. In one study, the prevalence of anxiety disorders and depression in patients with glioma determined using different diagnostic scales, such as Hospital Anxiety and Depression Scale and Quality of Life Core Questionnaire were 36.6–37.4 and 28.4–32.6% (93), respectively. Negative emotions in response to the tumor and the interplay between the immune, neural and psychological systems may lead to anxiety and depression in patients with glioma, and anxiety and depression have been reported to shorten the survival time in such patients (89,94,95). However, the precise cause remains unknown, and anxiety and depression continue to be important problems for patients with glioma.
A total of 190 patients with glioma treated with resection were included in a previous study. The hospital anxiety and depression scale (HADS) and the Zung self-rating anxiety scale (SAS) were used to assess anxiety, whereas the HADS and the Zung self-rating depression scale (SDS) were used to assess depression. All patients were monitored for 36 months or until death. According to the survival data, OS was calculated, and The HADS for anxiety, SAS and SDS were associated with a shorter OS, while the HADS for depression was not (93). Changes in the levels of receptor-interacting protein kinase 3, phosphorylated mixed lineage kinase domain-like protein, ferritin light chain and lipid peroxidation related to ferroptosis were demonstrated using western blotting and biochemical assays. The study reported a relationship between ferroptosis and depression, thereby identifying a possible therapeutic target for depression (93).
The presence of lipid peroxidation in psychiatric diseases has been demonstrated through clinical research. High lipid peroxidation rates were reported to increase the probability of treatment-resistant depression (96). Recently, edaravone was shown to ameliorate depression- and anxiety-like behaviors, oxidative stress and neuroinflammation in mouse models of depression induced by chronic social defeat stress (97).
GPX4-mediated ferroptosis may be modulated through underlying molecular mechanisms involving the sirtuin 1/Nrf2/heme oxygenase-1 pathway (Fig. 6). This previous study suggested that abnormal GPX4 expression is a potential mechanism underlying depression (97). Therefore, GPX4-mediated ferroptosis may be a promising target for treating major depressive disorder. Xiao Yao San, a traditional Chinese medicine, was reported to exert therapeutic effects by increasing the expression of GPX4 and other ferroptosis-related molecules in the hippocampus of depressed rats (97). A previous study revealed a downregulation of ERK levels in the brains of depressed individuals with suicidal tendencies (98). Alterations in the expression of PEBP1 may influence the ERK pathway, potentially contributing to the development of depression (98).
Figure 6.
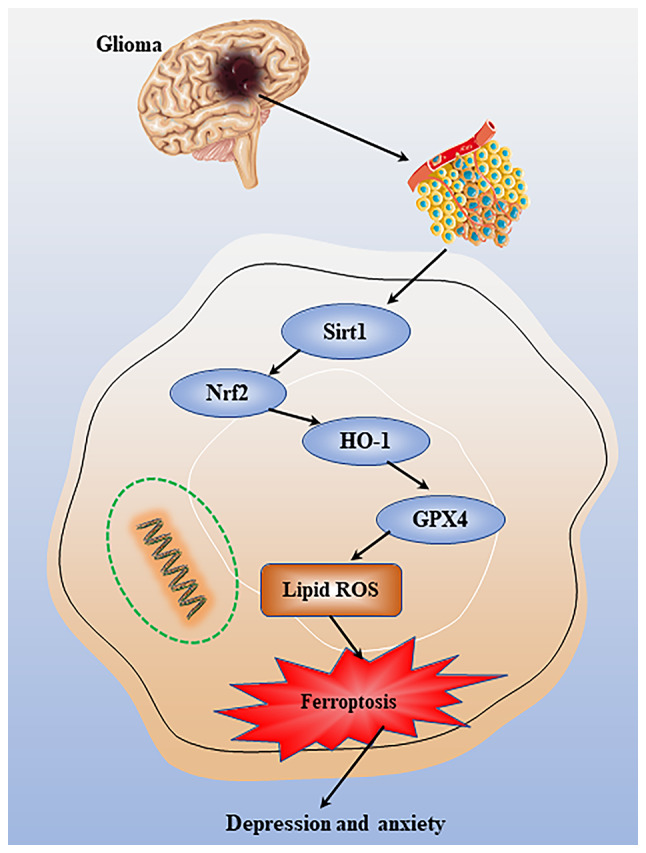
Mechanisms of anxiety and depression in glioma. Changes in microenvironmental oxidative stress in glioma can regulate ferroptotic death through a potential mechanism involving the Sirt1/Nrf2/HO-1 pathway, thereby affecting anxiety and depression. GPX, glutathione peroxidase; ROS, reactive oxygen species; Sirt1, sirtuin 1; Nrf2, nuclear factor erythroid 2-related factor 2; HO-1, heme oxygenase 1.
8. Conclusion
Ferroptosis occurs due to an imbalance between the body's antioxidant and oxidative mechanisms. Ferroptosis has been linked to numerous diseases and systemic conditions, including certain types of cancer, cardiovascular and digestive disorders. Moreover, ferroptosis serves a crucial role in the occurrence and development of gliomas, as well as the metabolic process of anxiety and depression, which can be caused by cancer. Ferroptosis-related biomarkers and long non-coding RNAs, such as AP003555.1 and AC000584.1, have been reported to be useful in predicting prognosis in patients with gliomas (99). Anxiety and depression can affect the prognosis of these patients, shortening their survival time. A recent study proposed that the WHO classification of gliomas is an independent risk factor for anxiety (93). However, no experimental study has elucidated the mechanism underlying glioma ferroptosis. More thorough research on ferroptosis is needed to assess its advantages and disadvantages, to enhance patient survival and quality of life, and to improve long-term clinical outcomes for patients with gliomas. The present review summarizes the research progress on ferroptosis in gliomas and its mechanistic relevance to anxiety and depression. For patients with gliomas, the focus should not only be on treatment methods, but also on their quality of life after being diagnosed, with the state of their mental health being the most concerning and a matter of societal interest. Patients with neuroglioma have a high incidence of anxiety and depression, markedly impacting their quality of life. Therefore, the evaluation of the mechanisms behind anxiety and depression in patients with glioma can provide a theoretical basis for the improvement of patient outcomes.
Acknowledgements
Not applicable.
Funding Statement
Funding: No funding was received.
Availability of data and materials
Not applicable.
Authors' contributions
YB wrote the majority of the manuscript. LM was involved in topic selection and wrote a draft of the review. ZY, WL and MJ edited the manuscript. All authors have read and approved the final manuscript. Data authentication is not applicable.
Ethics approval and consent to participate
Not applicable.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
References
- 1.Schaff LR, Mellinghoff IK. Glioblastoma and other primary brain malignancies in adults: A review. JAMA. 2023;329:574–587. doi: 10.1001/jama.2023.0023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.McNamara C, Mankad K, Thust S, Dixon L, Limback-Stanic C, D'Arco F, Jacques TS, Löbel U. 2021 WHO classification of tumours of the central nervous system: A review for the neuroradiologist. Neuroradiology. 2022;64:1919–1950. doi: 10.1007/s00234-022-03008-6. [DOI] [PubMed] [Google Scholar]
- 3.Szklener K, Mazurek M, Wieteska M, Waclawska M, Bilski M, Mandziuk S. New directions in the therapy of glioblastoma. Cancers (Basel) 2022;14:5377. doi: 10.3390/cancers14215377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Salvador GA. Iron in neuronal function and dysfunction. Biofactors. 2010;36:103–110. doi: 10.1002/biof.80. [DOI] [PubMed] [Google Scholar]
- 5.Chen S, Zhang Z, Zhang B, Huang Q, Liu Y, Qiu Y, Qiu Y, Long X, Wu M, Zhang Z. CircCDK14 promotes tumor progression and resists ferroptosis in glioma by regulating PDGFRA. Int J Biol Sci. 2022;18:841–857. doi: 10.7150/ijbs.66114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Jiao JT, Jiang C, Huang J, Dai MC, Wang C, Cheng C, Shao JF. Metabolic syndrome factors and risk of postoperative depression in high-grade glioma patients in a 1.5-year prospective study. Med Oncol. 2014;31:234. doi: 10.1007/s12032-014-0234-y. [DOI] [PubMed] [Google Scholar]
- 7.Tiller JW. Depression and anxiety. Med J Aust. 2013;199((S6)):S28–S31. doi: 10.5694/mja12.10628. [DOI] [PubMed] [Google Scholar]
- 8.Middeldorp CM, Cath DC, Van Dyck R, Boomsma DI. The co-morbidity of anxiety and depression in the perspective of genetic epidemiology. A review of twin and family studies. Psychol Med. 2005;35:611–624. doi: 10.1017/S003329170400412X. [DOI] [PubMed] [Google Scholar]
- 9.Maes M, Kubera M, Obuchowiczwa E, Goehler L, Brzeszcz J. Depression's multiple comorbidities explained by (neuro)inflammatory and oxidative & nitrosative stress pathways. Neuro Endocrinol Lett. 2011;32:7–24. [PubMed] [Google Scholar]
- 10.Bunevicius A, Deltuva VP, Tamasauskas A. Association of pre-operative depressive and anxiety symptoms with five-year survival of glioma and meningioma patients: A prospective cohort study. Oncotarget. 2017;8:57543–57551. doi: 10.18632/oncotarget.15743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Gathinji M, McGirt MJ, Attenello FJ, Chaichana KL, Than K, Olivi A, Weingart JD, Brem H, Quinones-Hinojosa A. Association of preoperative depression and survival after resection of malignant brain astrocytoma. Surg Neurol. 2009;71:299–303. doi: 10.1016/j.surneu.2008.07.016. discussion 303. [DOI] [PubMed] [Google Scholar]
- 12.Conrad M, Lorenz SM, Proneth B. Targeting ferroptosis: New hope for as-yet-incurable diseases. Trends Mol Med. 2021;27:113–122. doi: 10.1016/j.molmed.2020.08.010. [DOI] [PubMed] [Google Scholar]
- 13.Liu T, Zhu C, Chen X, Guan G, Zou C, Shen S, Wu J, Wang Y, Lin Z, Chen L, et al. Ferroptosis, as the most enriched programmed cell death process in glioma, induces immunosuppression and immunotherapy resistance. Neuro Oncol. 2022;24:1113–1125. doi: 10.1093/neuonc/noac033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Wang Z, Li R, Hou N, Zhang J, Wang T, Fan P, Ji C, Zhang B, Liu L, Wang Y, et al. PRMT5 reduces immunotherapy efficacy in triple-negative breast cancer by methylating KEAP1 and inhibiting ferroptosis. J Immunother Cancer. 2023;11:e006890. doi: 10.1136/jitc-2023-006890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Yan H, Talty R, Johnson CH. Targeting ferroptosis to treat colorectal cancer. Trends Cell Biol. 2023;33:185–188. doi: 10.1016/j.tcb.2022.11.003. [DOI] [PubMed] [Google Scholar]
- 16.Ramadori P, Gallage S, Heikenwalder MF. Unique tumour microenvironment: When ferroptosis activation boosts ICI of liver cancer. Gut. 2023;72:1639–1641. doi: 10.1136/gutjnl-2023-329472. [DOI] [PubMed] [Google Scholar]
- 17.Han Z, Wang H, Long J, Qiu Y, Xing XL. Establishing a prognostic model of ferroptosis- and immune-related signatures in kidney cancer: A study based on TCGA and ICGC databases. Front Oncol. 2022;12:931383. doi: 10.3389/fonc.2022.931383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Chen X, Li J, Kang R, Klionsky DJ, Tang D. Ferroptosis: Machinery and regulation. Autophagy. 2020;17:2054–2081. doi: 10.1080/15548627.2020.1810918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Dixon SJ, Lemberg KM, Lamprecht MR, Skouta R, Zaitsev EM, Gleason CE, Patel DN, Bauer AJ, Cantley AM, Yang WS, et al. Ferroptosis: An iron-dependent form of nonapoptotic cell death. Cell. 2012;149:1060–1072. doi: 10.1016/j.cell.2012.03.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Degterev A, Huang Z, Boyce M, Li Y, Jagtap P, Mizushima N, Cuny GD, Mitchison TJ, Moskowitz MA, Yuan J. Chemical inhibitor of nonapoptotic cell death with therapeutic potential for ischemic brain injury. Nat Chem Biol. 2005;1:112–119. doi: 10.1038/nchembio0905-234a. [DOI] [PubMed] [Google Scholar]
- 21.Liu Y, Shoji-Kawata S, Sumpter RM, Jr, Wei Y, Ginet V, Zhang L, Posner B, Tran KA, Green DR, Xavier RJ, et al. Autosis is a Na+, K+-ATPase-regulated form of cell death triggered by autophagy-inducing peptides, starvation, and hypoxia-ischemia. Proc Natl Acad Sci USA. 2013;110:20364–20371. doi: 10.1073/pnas.1319661110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Yang WS, SriRamaratnam R, Welsch ME, Shimada K, Skouta R, Viswanathan VS, Cheah JH, Clemons PA, Shamji AF, Clish CB, et al. Regulation of ferroptotic cancer cell death by GPX4. Cell. 2014;156:317–331. doi: 10.1016/j.cell.2013.12.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Xie Y, Hou W, Song X, Yu Y, Huang J, Sun X, Kang R, Tang D. Ferroptosis: Process and function. Cell Death Differ. 2016;23:369–379. doi: 10.1038/cdd.2015.158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Gunshin H, Mackenzie B, Berger UV, Gunshin Y, Romero MF, Boron WF, Nussberger S, Gollan JL, Hediger MA. Cloning and characterization of a mammalian proton-coupled metal-ion transporter. Nature. 1997;388:482–488. doi: 10.1038/41343. [DOI] [PubMed] [Google Scholar]
- 25.Lane DJ, Richardson DR. Chaperone turns gatekeeper: PCBP2 and DMT1 form an iron-transport pipeline. Biochem J. 2014;462:e1–e3. doi: 10.1042/BJ20140720. [DOI] [PubMed] [Google Scholar]
- 26.Hurrell R, Egli I. Iron bioavailability and dietary reference values. Am J Clin Nutr. 2010;91:1461S–1467S. doi: 10.3945/ajcn.2010.28674F. [DOI] [PubMed] [Google Scholar]
- 27.Ma S, Henson ES, Chen Y, Gibson SB. Ferroptosis is induced following siramesine and lapatinib treatment of breast cancer cells. Cell Death Dis. 2016;7:e2307. doi: 10.1038/cddis.2016.208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Gao M, Monian P, Quadri N, Ramasamy R, Jiang X. Glutaminolysis and transferrin regulate ferroptosis. Mol Cell. 2015;59:298–308. doi: 10.1016/j.molcel.2015.06.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Yanatori I, Richardson DR, Imada K, Kishi F. Iron export through the transporter ferroportin 1 is modulated by the iron chaperone PCBP2. J Biol Chem. 2016;291:17303–17318. doi: 10.1074/jbc.M116.721936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Lane DJ, Bae DH, Merlot AM, Sahni S, Richardson DR. Duodenal cytochrome b (DCYTB) in iron metabolism: An update on function and regulation. Nutrients. 2015;7:2274–2296. doi: 10.3390/nu7042274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Qiu A, Jansen M, Sakaris A, Min SH, Chattopadhyay S, Tsai E, Sandoval C, Zhao R, Akabas MH, Goldman ID. Identification of an intestinal folate transporter and the molecular basis for hereditary folate malabsorption. Cell. 2006;127:917–928. doi: 10.1016/j.cell.2006.09.041. [DOI] [PubMed] [Google Scholar]
- 32.White C, Yuan X, Schmidt PJ, Bresciani E, Samuel TK, Campagna D, Hall C, Bishop K, Calicchio ML, Lapierre A, et al. HRG1 is essential for heme transport from the phagolysosome of macrophages during erythrophagocytosis. Cell Metab. 2013;17:261–270. doi: 10.1016/j.cmet.2013.01.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Nishito Y, Kambe T. Absorption mechanisms of iron, copper, and zinc: An overview. J Nutr Sci Vitaminol (Tokyo) 2018;64:1–7. doi: 10.3177/jnsv.64.1. [DOI] [PubMed] [Google Scholar]
- 34.Cao JY, Dixon SJ. Mechanisms of ferroptosis. Cell Mol Life Sci. 2016;73:2195–2209. doi: 10.1007/s00018-016-2194-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Zhao J, Wang Y, Tao L, Chen L. Iron transporters and ferroptosis in malignant brain tumors. Front Oncol. 2022;12:861834. doi: 10.3389/fonc.2022.861834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Hu C, Tao L, Cao X, Chen L. The solute carrier transporters and the brain: Physiological and pharmacological implications. Asian J Pharm Sci. 2020;15:131–144. doi: 10.1016/j.ajps.2019.09.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Rouault TA, Cooperman S. Brain iron metabolism. Semin Pediatr Neurol. 2006;13:142–148. doi: 10.1016/j.spen.2006.08.002. [DOI] [PubMed] [Google Scholar]
- 38.Engelhardt B, Sorokin L. The blood-brain and the blood-cerebrospinal fluid barriers: Function and dysfunction. Semin Immunopathol. 2009;31:497–511. doi: 10.1007/s00281-009-0177-0. [DOI] [PubMed] [Google Scholar]
- 39.Skjorringe T, Moller LB, Moos T. Impairment of interrelated iron- and copper homeostatic mechanisms in brain contributes to the pathogenesis of neurodegenerative disorders. Front Pharmacol. 2012;3:169. doi: 10.3389/fphar.2012.00169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Li GJ, Choi BS, Wang X, Liu J, Waalkes MP, Zheng W. Molecular mechanism of distorted iron regulation in the blood-CSF barrier and regional blood-brain barrier following in vivo subchronic manganese exposure. Neurotoxicology. 2006;27:737–744. doi: 10.1016/j.neuro.2006.02.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Zheng W, Monnot AD. Regulation of brain iron and copper homeostasis by brain barrier systems: Implication in neurodegenerative diseases. Pharmacol Ther. 2012;133:177–188. doi: 10.1016/j.pharmthera.2011.10.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Qian ZM, Ke Y. Brain iron transport. Biol Rev Camb Philos Soc. 2019;94:1672–1684. doi: 10.1111/brv.12521. [DOI] [PubMed] [Google Scholar]
- 43.Burdo JR, Menzies SL, Simpson IA, Garrick LM, Garrick MD, Dolan KG, Haile DJ, Beard JL, Connor JR. Distribution of divalent metal transporter 1 and metal transport protein 1 in the normal and Belgrade rat. J Neurosci Res. 2001;66:1198–1207. doi: 10.1002/jnr.1256. [DOI] [PubMed] [Google Scholar]
- 44.Carlson ES, Tkac I, Magid R, O'Connor MB, Andrews NC, Schallert T, Gunshin H, Georgieff MK, Petryk A. Iron is essential for neuron development and memory function in mouse hippocampus. J Nutr. 2009;139:672–679. doi: 10.3945/jn.108.096354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Wong BX, Tsatsanis A, Lim LQ, Adlard PA, Bush AI, Duce JA. β-Amyloid precursor protein does not possess ferroxidase activity but does stabilize the cell surface ferrous iron exporter ferroportin. PLoS One. 2014;9:e114174. doi: 10.1371/journal.pone.0114174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Miyajima H. Aceruloplasminemia. Neuropathology. 2015;35:83–90. doi: 10.1111/neup.12149. [DOI] [PubMed] [Google Scholar]
- 47.Gaasch JA, Lockman PR, Geldenhuys WJ, Allen DD, Van der Schyf CJ. Brain iron toxicity: Differential responses of astrocytes, neurons, and endothelial cells. Neurochem Res. 2007;32:1196–1208. doi: 10.1007/s11064-007-9488-5. [DOI] [PubMed] [Google Scholar]
- 48.Huang R, Dong R, Wang N, He Y, Zhu P, Wang C, Lan B, Gao Y, Sun L. Adaptive changes allow targeting of ferroptosis for glioma treatment. Cell Mol Neurobiol. 2022;42:2055–2074. doi: 10.1007/s10571-021-01092-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Du H, Ren X, Bai J, Yang W, Gao Y, Yan S. Research progress of ferroptosis in adiposity-based chronic disease (ABCD) Oxid Med Cell Longev. 2022;2022:1052699. doi: 10.1155/2022/1052699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Shintoku R, Takigawa Y, Yamada K, Kubota C, Yoshimoto Y, Takeuchi T, Koshiishi I, Torii S. Lipoxygenase-mediated generation of lipid peroxides enhances ferroptosis induced by erastin and RSL3. Cancer Sci. 2017;108:2187–2194. doi: 10.1111/cas.13380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Yang WS, Kim KJ, Gaschler MM, Patel M, Shchepinov MS, Stockwell BR. Peroxidation of polyunsaturated fatty acids by lipoxygenases drives ferroptosis. Proc Natl Acad Sci USA. 2016;113:E4966–E4975. doi: 10.1073/pnas.1603244113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Luo Y, Tian G, Fang X, Bai S, Yuan G, Pan Y. Ferroptosis and its potential role in glioma: From molecular mechanisms to therapeutic opportunities. Antioxidants (Basel) 2022;11:2123. doi: 10.3390/antiox11112123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Zhou Y, Fang C, Xu H, Yuan L, Liu Y, Wang X, Zhang A, Shao A, Zhou D. Ferroptosis in glioma treatment: Current situation, prospects and drug applications. Front Oncol. 2022;12:989896. doi: 10.3389/fonc.2022.989896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Yuan H, Li X, Zhang X, Kang R, Tang D. Identification of ACSL4 as a biomarker and contributor of ferroptosis. Biochem Biophys Res Commun. 2016;478:1338–1343. doi: 10.1016/j.bbrc.2016.08.124. [DOI] [PubMed] [Google Scholar]
- 55.Chu B, Kon N, Chen D, Li T, Liu T, Jiang L, Song S, Tavana O, Gu W. ALOX12 is required for p53-mediated tumour suppression through a distinct ferroptosis pathway. Nat Cell Biol. 2019;21:579–591. doi: 10.1038/s41556-019-0305-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Tuo QZ, Lei P, Jackman KA, Li XL, Xiong H, Li XL, Liuyang ZY, Roisman L, Zhang ST, Ayton S, et al. Tau-mediated iron export prevents ferroptotic damage after ischemic stroke. Mol Psychiatry. 2017;22:1520–1530. doi: 10.1038/mp.2017.171. [DOI] [PubMed] [Google Scholar]
- 57.Shin D, Lee J, You JH, Kim D, Roh JL. Dihydrolipoamide dehydrogenase regulates cystine deprivation-induced ferroptosis in head and neck cancer. Redox Biol. 2020;30:101418. doi: 10.1016/j.redox.2019.101418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Detivaud L, Island ML, Jouanolle AM, Ropert M, Bardou-Jacquet E, Le Lan C, Mosser A, Leroyer P, Deugnier Y, David V, et al. Ferroportin diseases: Functional studies, a link between genetic and clinical phenotype. Hum Mutat. 2013;34:1529–1536. doi: 10.1002/humu.22396. [DOI] [PubMed] [Google Scholar]
- 59.Luo M, Wu L, Zhang K, Wang H, Zhang T, Gutierrez L, O'Connell D, Zhang P, Li Y, Gao T, et al. miR-137 regulates ferroptosis by targeting glutamine transporter SLC1A5 in melanoma. Cell Death Differ. 2018;25:1457–1472. doi: 10.1038/s41418-017-0053-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Dai C, Chen X, Li J, Comish P, Kang R, Tang D. Transcription factors in ferroptotic cell death. Cancer Gene Ther. 2020;27:645–656. doi: 10.1038/s41417-020-0170-2. [DOI] [PubMed] [Google Scholar]
- 61.Anandhan A, Dodson M, Schmidlin CJ, Liu P, Zhang DD. Breakdown of an ironclad defense system: The critical role of NRF2 in mediating ferroptosis. Cell Chem Biol. 2020;27:436–447. doi: 10.1016/j.chembiol.2020.03.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Liu XB, Yang F, Haile DJ. Functional consequences of ferroportin 1 mutations. Blood Cells Mol Dis. 2005;35:33–46. doi: 10.1016/j.bcmd.2005.04.005. [DOI] [PubMed] [Google Scholar]
- 63.Taniguchi R, Kato HE, Font J, Deshpande CN, Wada M, Ito K, Ishitani R, Jormakka M, Nureki O. Outward- and inward-facing structures of a putative bacterial transition-metal transporter with homology to ferroportin. Nat Commun. 2015;6:8545. doi: 10.1038/ncomms9545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Galaris D, Barbouti A, Pantopoulos K. Iron homeostasis and oxidative stress: An intimate relationship. Biochim Biophys Acta Mol Cell Res. 2019;1866:118535. doi: 10.1016/j.bbamcr.2019.118535. [DOI] [PubMed] [Google Scholar]
- 65.Pardridge WM. Drug transport across the blood-brain barrier. J Cereb Blood Flow Metab. 2012;32:1959–1972. doi: 10.1038/jcbfm.2012.126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Wang H, An P, Xie E, Wu Q, Fang X, Gao H, Zhang Z, Li Y, Wang X, Zhang J, et al. Characterization of ferroptosis in murine models of hemochromatosis. Hepatology. 2017;66:449–465. doi: 10.1002/hep.29117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Badgley MA, Kremer DM, Maurer HC, DelGiorno KE, Lee HJ, Purohit V, Sagalovskiy IR, Ma A, Kapilian J, Firl CEM, et al. Cysteine depletion induces pancreatic tumor ferroptosis in mice. Science. 2020;368:85–89. doi: 10.1126/science.aaw9872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Ingold I, Berndt C, Schmitt S, Doll S, Poschmann G, Buday K, Roveri A, Peng X, Porto Freitas F, Seibt T, et al. Selenium utilization by GPX4 is required to prevent hydroperoxide-induced ferroptosis. Cell. 2018;172:409–422. e21. doi: 10.1016/j.cell.2017.11.048. [DOI] [PubMed] [Google Scholar]
- 69.Alim I, Caulfield JT, Chen Y, Swarup V, Geschwind DH, Ivanova E, Seravalli J, Ai Y, Sansing LH, Ste Marie EJ, et al. Selenium drives a transcriptional adaptive program to block ferroptosis and treat stroke. Cell. 2019;177:1262–1279. e25. doi: 10.1016/j.cell.2019.03.032. [DOI] [PubMed] [Google Scholar]
- 70.Doll S, Freitas FP, Shah R, Aldrovandi M, da Silva MC, Ingold I, Goya Grocin A, Xavier da Silva TN, Panzilius E, Scheel CH, et al. FSP1 is a glutathione-independent ferroptosis suppressor. Nature. 2019;575:6936–698. doi: 10.1038/s41586-019-1707-0. [DOI] [PubMed] [Google Scholar]
- 71.Bersuker K, Hendricks JM, Li Z, Magtanong L, Ford B, Tang PH, Roberts MA, Tong B, Maimone TJ, Zoncu R, et al. The CoQ oxidoreductase FSP1 acts parallel to GPX4 to inhibit ferroptosis. Nature. 2019;575:688–692. doi: 10.1038/s41586-019-1705-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Wang Z, Ding Y, Wang X, Lu S, Wang C, He C, Wang L, Piao M, Chi G, Luo Y, Ge P. Pseudolaric acid B triggers ferroptosis in glioma cells via activation of Nox4 and inhibition of xCT. Cancer Lett. 2018;428:21–33. doi: 10.1002/cncr.30911. [DOI] [PubMed] [Google Scholar]
- 73.Wu Y, Zhao Y, Yang HZ, Wang YJ, Chen Y. HMGB1 regulates ferroptosis through Nrf2 pathway in mesangial cells in response to high glucose. Biosci Rep. 2021;41:BSR20202924. doi: 10.1042/BSR20202924. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Fan Z, Wirth AK, Chen D, Wruck CJ, Rauh M, Buchfelder M, Savaskan N. Nrf2-Keap1 pathway promotes cell proliferation and diminishes ferroptosis. Oncogenesis. 2017;6:e371. doi: 10.1038/oncsis.2017.65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Roh JL, Kim EH, Jang H, Shin D. Nrf2 inhibition reverses the resistance of cisplatin-resistant head and neck cancer cells to artesunate-induced ferroptosis. Redox Biol. 2017;11:254–262. doi: 10.1016/j.redox.2016.12.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Sun X, Ou Z, Chen R, Niu X, Chen D, Kang R, Tang D. Activation of the p62-Keap1-NRF2 pathway protects against ferroptosis in hepatocellular carcinoma cells. Hepatology. 2016;63:173–184. doi: 10.1002/hep.28251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Chen D, Fan Z, Rauh M, Buchfelder M, Eyupoglu IY, Savaskan N. ATF4 promotes angiogenesis and neuronal cell death and confers ferroptosis in a xCT-dependent manner. Oncogene. 2017;36:5593–5608. doi: 10.1038/onc.2017.146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Zhao X, Zhou M, Yang Y, Luo M. The ubiquitin hydrolase OTUB1 promotes glioma cell stemness via suppressing ferroptosis through stabilizing SLC7A11 protein. Bioengineered. 2021;12:12636–12645. doi: 10.1080/21655979.2021.2011633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Yi R, Wang H, Deng C, Wang X, Yao L, Niu W, Fei M, Zhaba W. Dihydroartemisinin initiates ferroptosis in glioblastoma through GPX4 inhibition. Biosci Rep. 2020;40:BSR20193314. doi: 10.1042/BSR20193314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Song Q, Peng S, Sun Z, Heng X, Zhu X. Temozolomide drives ferroptosis via a DMT1-Dependent pathway in glioblastoma cells. Yonsei Med J. 2021;62:843–849. doi: 10.3349/ymj.2021.62.9.843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Zhang Y, Tan H, Daniels JD, Zandkarimi F, Liu H, Brown LM, Uchida K, O'Connor OA, Stockwell BR. Imidazole ketone erastin induces ferroptosis and slows tumor growth in a mouse lymphoma model. Cell Chem Biol. 2019;26:623–633. e9. doi: 10.1016/j.chembiol.2019.01.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Eaton JK, Furst L, Ruberto RA, Moosmayer D, Hilpmann A, Ryan MJ, Zimmermann K, Cai LL, Niehues M, Badock V, et al. Selective covalent targeting of GPX4 using masked nitrile-oxide electrophiles. Nat Chem Biol. 2020;16:497–506. doi: 10.1038/s41589-020-0501-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Sukseree S, Schwarze UY, Gruber R, Gruber F, Quiles Del Rey M, Mancias JD, Bartlett JD, Tschachler E, Eckhart L. ATG7 is essential for secretion of iron from ameloblasts and normal growth of murine incisors during aging. Autophagy. 2020;16:1851–1857. doi: 10.1080/15548627.2019.1709764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Zhang Y, Kong Y, Ma Y, Ni S, Wikerholmen T, Xi K, Zhao F, Zhao Z, Wang J, Huang B, et al. Loss of COPZ1 induces NCOA4 mediated autophagy and ferroptosis in glioblastoma cell lines. Oncogene. 2021;40:1425–1439. doi: 10.1038/s41388-020-01622-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Santini ZI, Jose PE, York Cornwell E, Koyanagi A, Nielsen L, Hinrichsen C, Meilstrup C, Madsen KR, Koushede V. Social disconnectedness, perceived isolation, and symptoms of depression and anxiety among older Americans (NSHAP): A longitudinal mediation analysis. Lancet Public Health. 2020;5:e62–e70. doi: 10.1016/S2468-2667(19)30230-0. [DOI] [PubMed] [Google Scholar]
- 86.Forero DA, Guio-Vega GP, Gonzalez-Giraldo Y. A comprehensive regional analysis of genome-wide expression profiles for major depressive disorder. J Affect Disord. 2017;218:86–92. doi: 10.1016/j.jad.2017.04.061. [DOI] [PubMed] [Google Scholar]
- 87.Tang W, Lu Y, Xu J. Post-traumatic stress disorder, anxiety and depression symptoms among adolescent earthquake victims: Comorbidity and associated sleep-disturbing factors. Soc Psychiatry Psychiatr Epidemiol. 2018;53:1241–1251. doi: 10.1007/s00127-018-1576-0. [DOI] [PubMed] [Google Scholar]
- 88.Gialluisi A, Bonaccio M, Di Castelnuovo A, Costanzo S, De Curtis A, Sarchiapone M, Cerletti C, Donati MB, de Gaetano G, Iacoviello L, Moli-Sani Study Investigators Lifestyle and biological factors influence the relationship between mental health and low-grade inflammation. Brain Behav Immun. 2020;85:4–13. doi: 10.1016/j.bbi.2019.04.041. [DOI] [PubMed] [Google Scholar]
- 89.Treudler R, Zeynalova S, Riedel-Heller SG, Zuelke AE, Roehr S, Hinz A, Glaesmer H, Kage P, Loeffler M, Simon JC. Depression, anxiety and quality of life in subjects with atopic eczema in a population-based cross-sectional study in Germany. J Eur Acad Dermatol Venereol. 2020;34:810–816. doi: 10.1111/jdv.16148. [DOI] [PubMed] [Google Scholar]
- 90.Singer S, Roick J, Danker H, Kortmann RD, Papsdorf K, Taubenheim S, Renovanz M, Jähne K, Meixensberger J. Psychiatric co-morbidity, distress, and use of psycho-social services in adult glioma patients-a prospective study. Acta Neurochir (Wien) 2018;160:1187–1194. doi: 10.1007/s00701-018-3527-7. [DOI] [PubMed] [Google Scholar]
- 91.Louis DN, Perry A, Reifenberger G, von Deimling A, Figarella-Branger D, Cavenee WK, Ohgaki H, Wiestler OD, Kleihues P, Ellison DW. The 2016 World health organization classification of tumors of the central nervous system: A summary. Acta Neuropathol. 2016;131:803–820. doi: 10.1007/s00401-016-1545-1. [DOI] [PubMed] [Google Scholar]
- 92.Ostrom QT, Gittleman H, Fulop J, Liu M, Blanda R, Kromer C, Wolinsky Y, Kruchko C, Barnholtz-Sloan JS. CBTRUS statistical report: Primary brain and central nervous system tumors diagnosed in the United States in 2008–2012. Neuro Oncol. 2015;17((Suppl 4)):iv1–iv62. doi: 10.1093/neuonc/nov189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Hao A, Huang J, Xu X. Anxiety and depression in glioma patients: Prevalence, risk factors, and their correlation with survival. Ir J Med Sci. 2021;190:1155–1164. doi: 10.1007/s11845-020-02374-5. [DOI] [PubMed] [Google Scholar]
- 94.Young K, Singh G. Biological mechanisms of cancer-induced depression. Front Psychiatry. 2018;9:299. doi: 10.3389/fpsyt.2018.00299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Satin JR, Linden W, Phillips MJ. Depression as a predictor of disease progression and mortality in cancer patients: A meta-analysis. Cancer. 2009;115:5349–5361. doi: 10.1002/cncr.24561. [DOI] [PubMed] [Google Scholar]
- 96.Sawangjit A, Oyanedel CN, Niethard N, Salazar C, Born J, Inostroza M. The hippocampus is crucial for forming non-hippocampal long-term memory during sleep. Nature. 2018;564:109–113. doi: 10.1038/s41586-018-0716-8. [DOI] [PubMed] [Google Scholar]
- 97.Dang R, Wang M, Li X, Wang H, Liu L, Wu Q, Zhao J, Ji P, Zhong L, Licinio J, Xie P. Edaravone ameliorates depressive and anxiety-like behaviors via Sirt1/Nrf2/HO-1/Gpx4 pathway. J Neuroinflammation. 2022;19:41. doi: 10.1186/s12974-022-02400-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Jiao H, Yang H, Yan Z, Chen J, Xu M, Jiang Y, Liu Y, Xue Z, Ma Q, Li X, Chen J. Traditional Chinese formula xiaoyaosan alleviates depressive-like behavior in CUMS Mice by Regulating PEBP1-GPX4-Mediated Ferroptosis in the Hippocampus. Neuropsychiatr Dis Treat. 2021;17:1001–1019. doi: 10.2147/NDT.S302443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Erratum to: path-03. Ferroptosis-related long non-coding rna signatures predict prognosis in patients with glioma. Neuro Oncol. 2022;24:2010. doi: 10.1093/neuonc/noac104. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Not applicable.