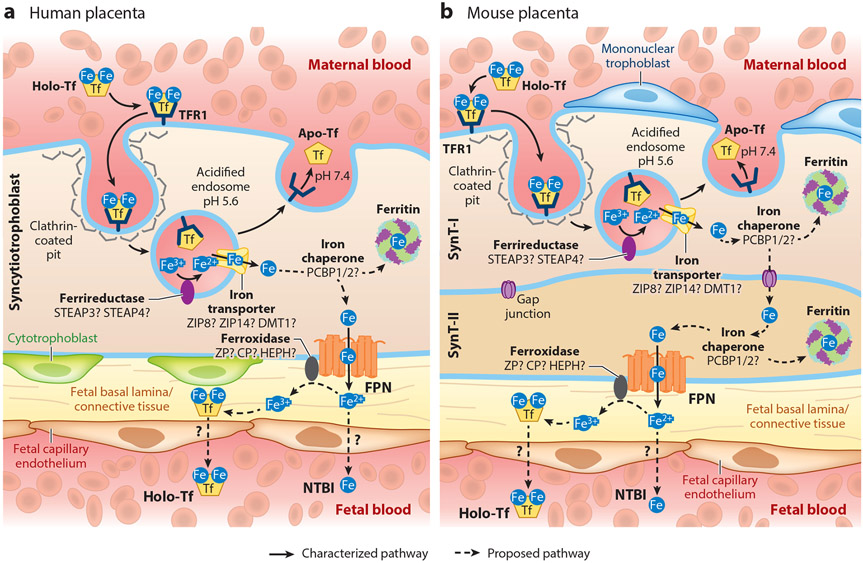
Figure 2.
Iron trafficking across the syncytiotrophoblast. (a) Human placenta, with a single layer of syncytiotrophoblast. (b) Mouse placenta, with two syncytiotrophoblast layers (SynT-I and SynT-II). In both humans and mice, transferrin-bound iron (holo-Tf) from the maternal circulation binds to transferrin receptor TFR1, expressed on the apical membrane of the placental syncytiotrophoblast (SynT-I in mice). The iron-transferrin-receptor complex is internalized via clathrin-mediated endocytosis, and ferric iron (Fe3+) is released from transferrin (Tf) in acidified endosomes. The apo-Tf/TFR1 complex is recycled back to the cell surface. Fe3+ in the endosome is thought to be reduced to ferrous iron (Fe2+) by a ferrireductase and exported into the cytoplasm through an endosomal iron transporter. Cytoplasmic Fe may be chaperoned, possibly by PCBP1 or PCBP2, either to ferritin for storage or to ferroportin (FPN) on the basal membrane (SynT-II in mice) for export toward the fetal circulation. In the mouse placenta (b), it is unknown how Fe is transported from SynT-I to SynT-II, but it likely occurs through gap junctions. The fate of iron following export through ferroportin is unclear; it may enter the fetal circulation as nontransferrin bound iron (NTBI) or be oxidized to Fe3+ by ferroxidases and loaded onto transferrin prior to reaching the fetal circulation. Figure adapted with permission from Reference 114.