Figure 1. ProP-PD screening identified a specific OGT binding motif.
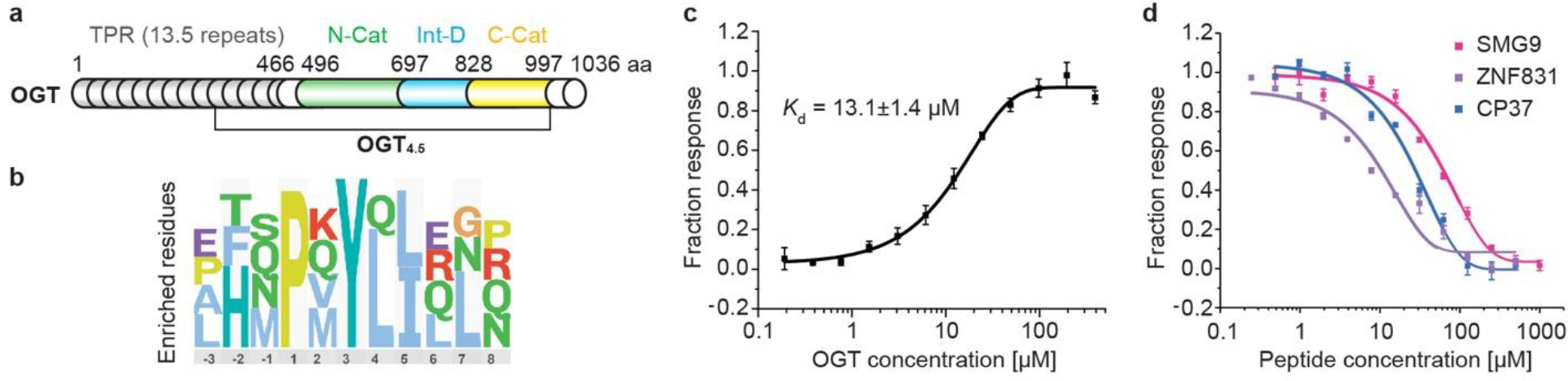
(a) Domain schematic of full-length OGT with tetratricopeptide repeat (TPR) domain in gray, N-terminal catalytic (N-Cat) domain in green, intervening domain (Int-D) in blue, and C-terminal catalytic (C-Cat) domain in yellow. The crystallization construct OGT4.5 with only 4.5 of 13.5 TPR repeats was also shown. (b) Sequence logo of highly enriched peptides from both OGT and OGT4.5 ProP-PD screens, aligned to the PxYx[I/L] motif. (c) Microscale thermophoresis (MST) binding assay of SMG9 peptide with OGT, n=3. (d) Competitive fluorescence polarization (FP) binding assay with fluorescently labeled 5-FAM-SMG9 peptide competing with unmodified SMG9, ZNF831, and consensus peptide 37 (CP37), for OGT binding, n=3. Data are presented as mean values +/− SD of three replicates.
