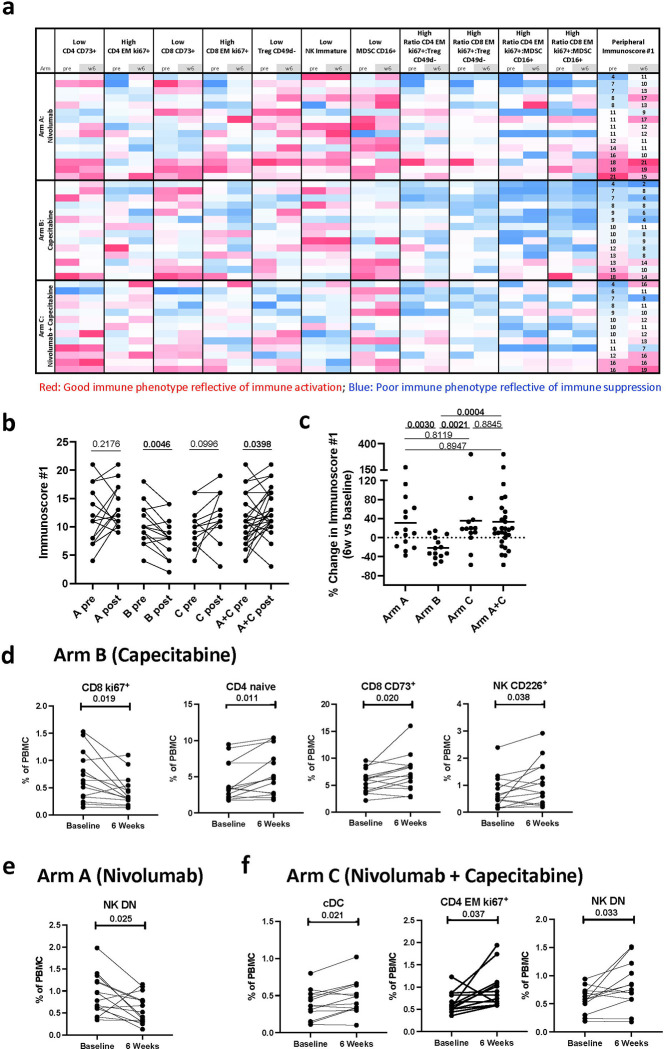
Fig. 1. Changes in peripheral immunoscore #1, and other immune cell subsets after 6 weeks of therapy.
(A) Heatmap representing the frequency at landmark and 6 weeks of refined classic peripheral blood mononuclear (PBMC) subsets of cell types reflecting known function (Supplementary Table 3) that were used to generate an immunoscore (peripheral immunoscore #1) in patients enrolled in arms A (n=15), B (n=14), C (n=13), and arms A and C combined (n=28). Each row corresponds to one patient. Peripheral immunoscore #1 is the sum of points assigned to each subset based on tertile distribution as previously described 42. (B) The peripheral immunoscore #1 calculated in A before and after 6 weeks of therapy in each treatment arm and arms A and C combined. (C) Comparison of the percent change after 6 weeks vs baseline in the peripheral immunoscore in each arm and arms A and C combined. p values are shown; p values were calculated by a two tailed Wilcoxon signed-rank test in B and a two tailed Mann-Whitney test in C, and no adjustments were made for multiple comparisons. Additional immune changes in the peripheral immune profile after 6 weeks of treatment in patients treated with (D) capecitabine (n=14), (E) nivolumab (n=15), and (F) nivolumab plus capecitabine (n=13). For D-F, changes in 10 classic PBMC cell types and 148 refined subsets reflective of maturation and function were analyzed with no adjustments made for multiple comparisons. Notable subsets with significant changes at post timepoints vs. landmark are displayed in D-F and include those with p < 0.05 (calculated by a two tailed Wilcoxon signed-rank test), difference in medians > 0.05, and ≥ 50% of patients having a > 25% change.
cDC, conventional dendritic cell; DN, double negative; EM, effector memory, NK, natural killer cells; Treg, regulatory T cell; MDSC, myeloid-derived suppressor cells; PBMC, peripheral blood mononuclear cells. Source data are provided as a source data file.