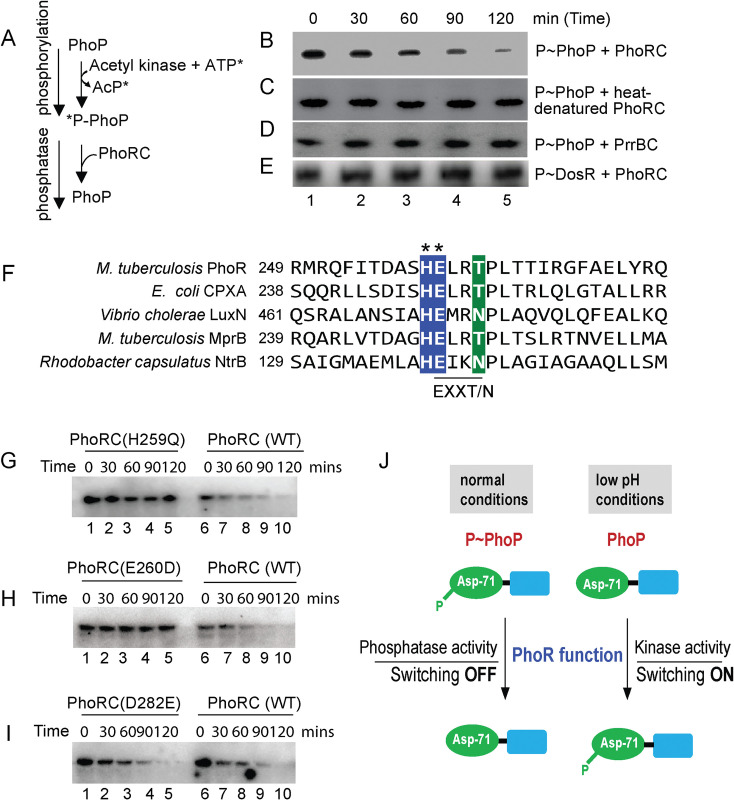
Fig 4. Identifying motif/residues responsible for phosphatase function of PhoR.
(A) Scheme showing phosphorylation of PhoP by γ-32P-ATP and acetyl kinase and dephosphorylation of P-PhoP. (B-E) Time-dependent dephosphorylation of P-PhoP was examined by adding recombinant PhoRC (B), heat-denatured PhoRC (C), and purified PrrBC (D) as described in the Methods. (E) To examine specificity of newly-identified PhoR phosphatase activity, P~DosR was used as a substrate to examine possible dephosphorylation by adding purified PhoRC. The reactions were followed by SDS-PAGE analyses and digited by a phosphorimager. Each data is representative of at least two independent experiments. (F) DHp domain of PhoR was aligned with other SKs, which displayed phosphatase activity. The most conserved residues of the EXXT/N motif are indicated by asterisks. (G-I) To investigate the effect of mutations on the phosphatase function of PhoRC, purified WT and mutant proteins were incubated with P~PhoP for indicated times as described in Fig 4B. In all cases, reactions were followed by SDS-PAGE analyses, and digitized by phosphorimager. (J) Schematic model showing balancing act of dual functioning of PhoR as a kinase (activation) and phosphatase (repression). Mutant PhoR protein, defective for kinase activity, fails to phosphorylate PhoP and thereby impact acid-inducible expression of the PhoP regulon. In contrast, phosphatase activity of PhoR dephosphorylates P~PhoP either to reverse active mycobacterial PhoP regulon in the absence of an inducing signal or to prevent unnecessary ‘triggering on’ of PhoP regulon. In summary, PhoR regulates net phosphorylation of PhoP by its dual functioning (kinase/phosphatase) to control context-sensitive expression of the PhoP regulon.