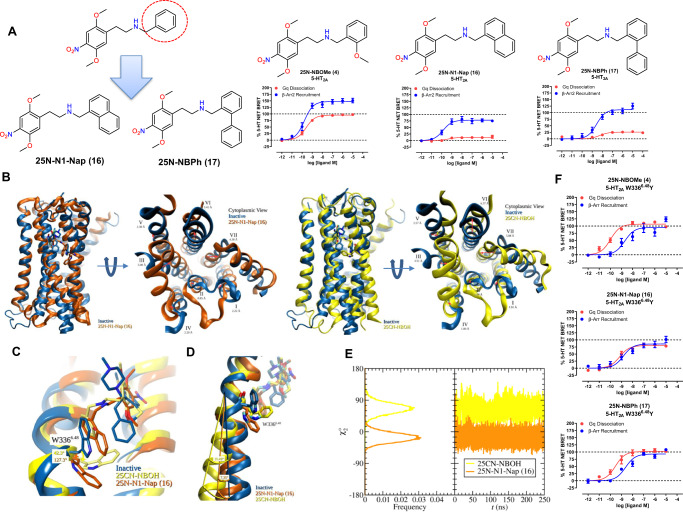
Fig. 3. Structure-based design of β-arrestin-biased 5-HT2A agonists.
A Effect of the larger N-substituted 25N analogs 25N-N1-Nap (16) and 25N-NBPh (17) on 5-HT2A receptor Gq dissociation (red) and β-arrestin2 (blue) recruitment. Data represent the mean and SEM from three independent experiments performed at 37 °C with 60-minute compound incubation. B Outward pivot of TM6 for 25CN-NBOH and 25N-N1-Nap (16) MD simulations relative to the inactive 5-HT2A receptor structure with final frame shown as representative. Note the intermediate TM6 tilt angle with 25N-N1-Nap (16) relative to that of 25CN-NBOH. C Change in W3666.48 toggle switch χ2 angle. χ2 angle given is the absolute difference in peak angle relative to the inactive state. Final frame shown as representative. D Distribution and time dependence W3666.48 χ2 angle of 25CN-NBOH (yellow) and 25N-N1-Nap (16) (orange) simulations. E Effect of 5-HT2A receptor W3366.48Y mutation on 25N-NBOMe (4), 25N-NBPh (17), and 25N-N1-Nap (16) Gq dissociation (red) versus β-arrestin2 (blue) recruitment activities. Data represent the mean and SEM from three independent experiments performed at 37 °C with 60-minute compound incubation. Source data are provided as a Source Data file.