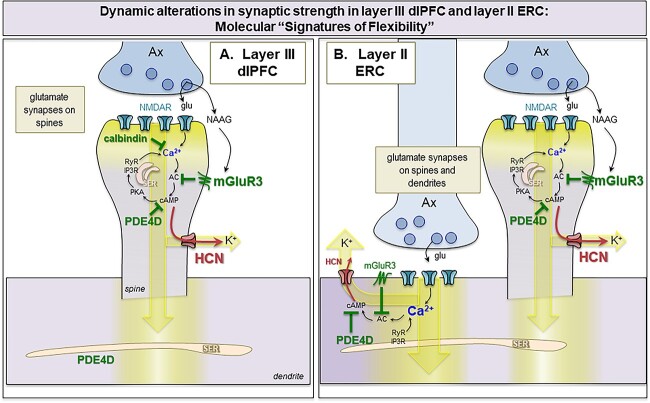
Fig. 13.
Hypothetic model of “Signature of flexibility” in higher-order association cortices in primates. (A) Schematic diagram of the dlPFC layer III dendritic spines that partake in recurrent excitatory microcircuits subserving working memory. Our previous findings have revealed that glutamatergic synapses on dendritic spines in layer III of the dlPFC express feedforward Ca2+-cAMP-K+ channel signaling to dynamically gate network inputs, providing a “signature of flexibility” (Arnsten et al. 2021c). In these microcircuits, neurotransmission depends on NMDAR (with GluN2A and GluN2B subunits), and the molecular machinery for cAMP-PKA to magnify Ca2+ signaling needed to sustain persistent firing. This includes cAMP-PKA amplification of internal Ca2+ release from the SER spine apparatus, which in turn increases cAMP production, leading to feedforward cAMP-Ca2+ signaling. dlPFC layer III dendritic spines also express K+ channels (e.g. HCN1 cation channels, Slack K+ channels, KCNQ2) that are opened by cAMP-PKA signaling to provide dynamic changes in network connectivity. Under healthy conditions in the young adult dlPFC, these intracellular signaling pathways are tightly regulated by the calcium binding protein, calbindin, the phosphodiesterases PDE4A/D, which are anchored to the SER spine apparatus and catabolize cAMP, and receptors that inhibit cAMP production, e.g. mGluR3. In primate layer III dlPFC, mGluR3 are concentrated on dendritic spines, where they provide inhibitory regulation of cAMP-K+ channel signaling, strengthening connectivity and markedly enhancing delay cell firing. PDE4s are also found in dendrites near mitochondria, positioned to regulate cAMP drive on Ca2+ release from the SER into mitochondria (not shown). (B) The current study examined whether a similar “signature of flexibility” is expressed in ERC layer II circuits, examining the subcellular localization of PDE4D, HCN1 channels, and mGluR3. Our findings showed convergent anatomical patterns to dlPFC, with PDE4D and mGluR3 positioned to regulate internal calcium release near glutamate synapses, and nearby HCN1 channels to provide dynamic changes in synaptic strength. In contrast to dlPFC, this constellation was seen on dendrites as well as dendritic spines, consistent with the larger numbers of glutamate synapses on dendritic shafts in layer II ERC. As layer II ERC stellate cells do not express calbindin, even when young, they may be particularly vulnerable to magnified calcium actions and ensuing tau pathology with advanced age in AD.