Figure 2: Key pathways active in quiescent stem cell processes.
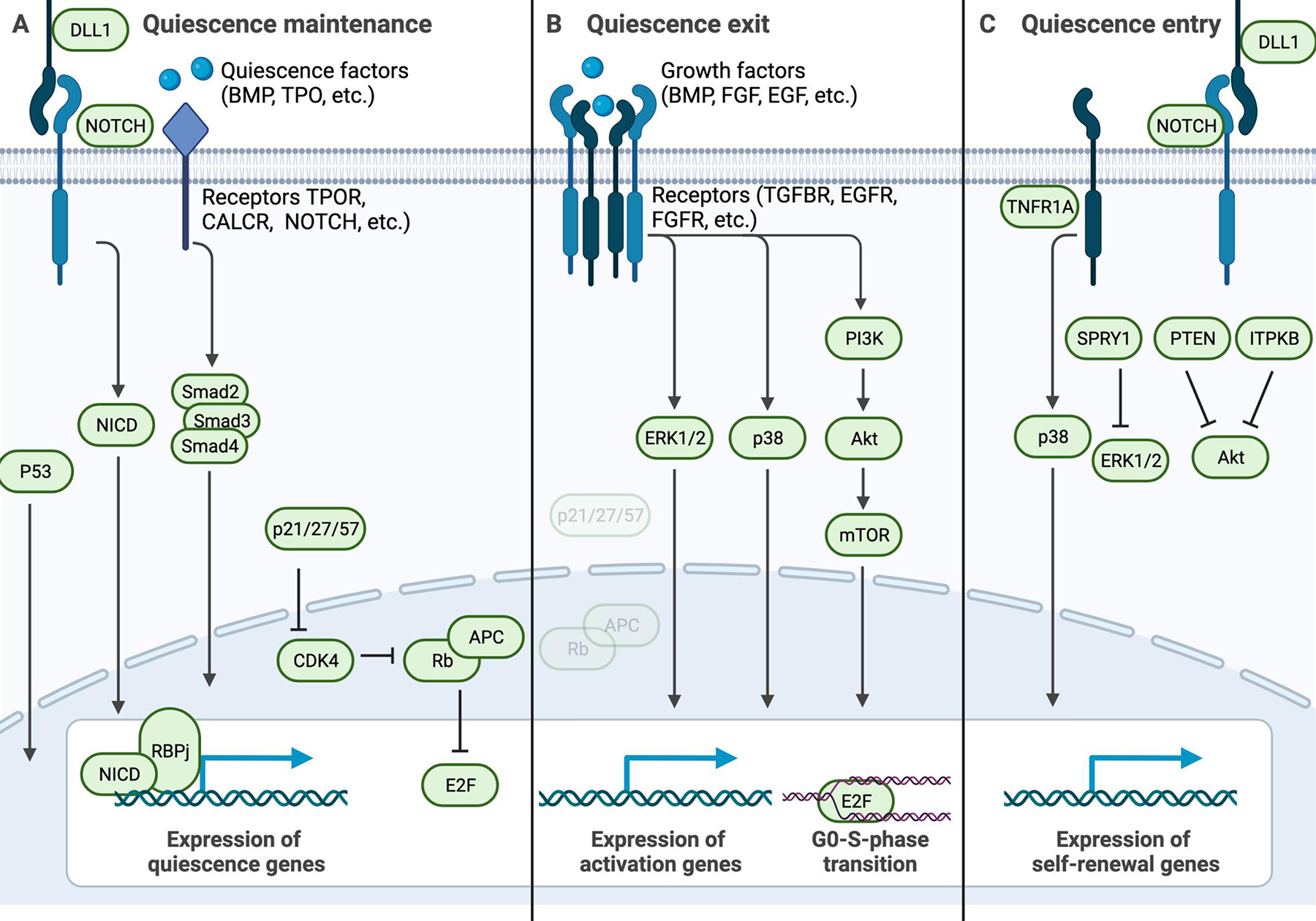
Schematic highlighting key signaling pathways that are active during quiescence maintenance, exit, or entry. A) Signals from the niche to the quiescent stem cell that maintain the quiescencent state, such as ligands for Notch receptors including DLL1 (delta-like protein 1), and quiescence factors including BMP (bone morphogenic protein), TPO (thrombopoietin), calcitonin, and COLV (collagen V) to their respective receptors NOTCH, BMPRII (bone morphogenic protein receptor 2), TPOR (thrombopoietin receptor), and CALCR (calcitonin receptor). Such receptor-mediated signaling induces gene expression patterns that maintain the quiescent state, via signalling intermediates such as NICD-RBPj, and SMAD2–4. The expression of cell-cycle inhibitors such as p21 similarly invokes quiescence maintenance via inhibition of CDK4 or CDK6, which ensures that APC/C (anaphase promoting complex/cyclosome) and Rb (retinoblastoma) block E2F and S-phase entry. B) In response to growth factors, combined with the loss of signals listed in panel (A), phosphorylation cascades such as the PI3K-AKT pathway and the p38 MAP kinase pathway induce quiescence exit and cell cycle entry. C) To exit the cell cycle and enter the quiescent state, stem cells interact with NOTCH ligands and suppress the phosphorylation cascades listed in panel (B). In activated NSCs, TNFR1 (tumor necrosis factor 1 receptor) signaling through p38 MAP kinase is key for re-entry into the quiescent state. PTEN (phosphatase and tensin homolog), MEK1 (MAPK/ERK kinase 1), and ITPKB (inositoltrisphosphate 3-kinase b) inhibit AKT, while Sprouty1 (SPRY1) suppresses ERK signaling to stop mitogenic signaling.
NICD, notch intracellular domain; RBPj, recombination signal binding protein for immunoglobulin kappa j region; CDK4/6, cyclin dependent kinase 4/6; PI3K, phosphatidylinositol-3 kinase.
