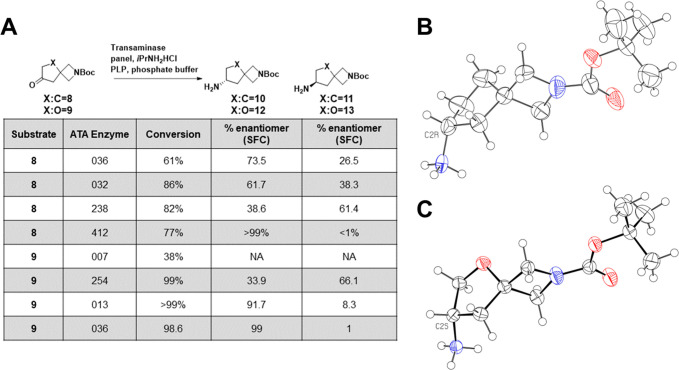
Figure 2.
Enzymatic synthesis of key chiral diamine intermediates. (A) Screen of transaminases identifies promising enzymes for further reaction development. (B) Crystal structure of 10, showing the R-configuration at C2. Compound was crystallized as the hydrochloride and is conformationally disordered over three sites (ratio 0.56:0.24:0.20). Anion and minor conformations are omitted for clarity. Ellipsoids are at 50% probability, and the radii of hydrogen atoms arbitrary. (C) Crystal structure of 12, showing the S-configuration at C2. Compound was crystallized as the hydrochloride. Disordered chloride anion is omitted for clarity. Ellipsoids at 50% probability, radii of hydrogen atoms arbitrary.