Figure 1.
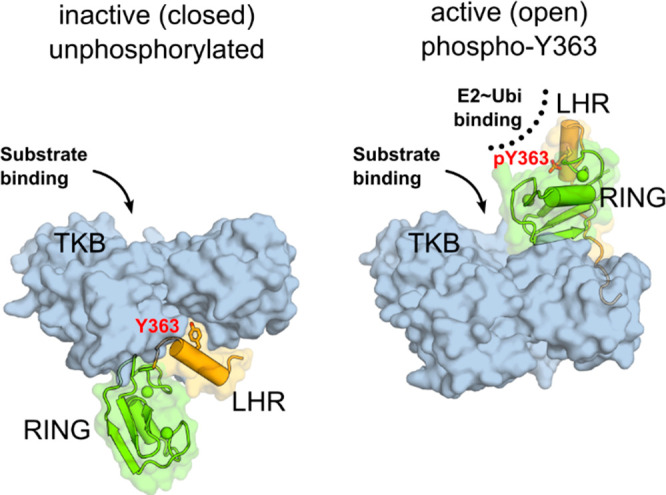
Cbl-b regulation by phosphorylation of Y363. Left: Unphosphorylated, inactive Cbl-b adopts a “closed” conformation with Y363, shown as sticks, buried from solvent. The linker helix region (LHR, orange) and the RING domain (green) are sequestered away from the substrate binding site on the TKB domain (blue surface). Right: Phosphorylation of Y363 induces the active (open) conformation, with the RING domain and phospho-Y363 in the LHR poised to recruit and activate ubiquitin-charged E2 conjugating enzyme (PDB code 3ZNI).28
