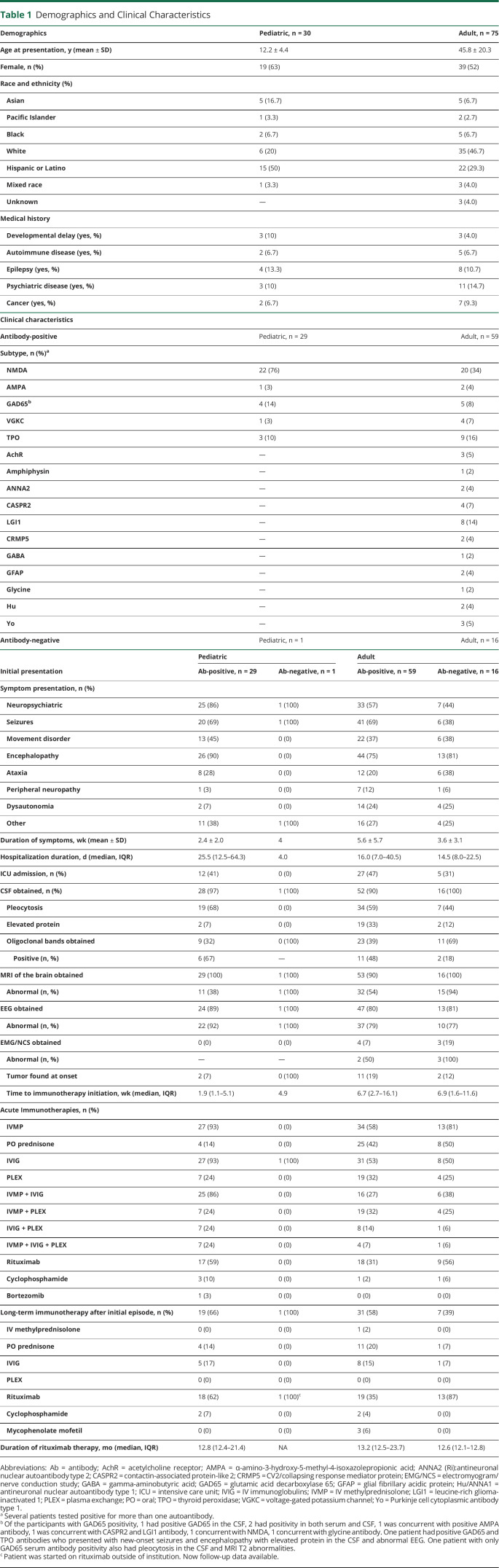
Table 1.
Demographics and Clinical Characteristics
| Demographics | Pediatric, n = 30 | Adult, n = 75 |
| Age at presentation, y (mean ± SD) | 12.2 ± 4.4 | 45.8 ± 20.3 |
| Female, n (%) | 19 (63) | 39 (52) |
| Race and ethnicity (%) | ||
| Asian | 5 (16.7) | 5 (6.7) |
| Pacific Islander | 1 (3.3) | 2 (2.7) |
| Black | 2 (6.7) | 5 (6.7) |
| White | 6 (20) | 35 (46.7) |
| Hispanic or Latino | 15 (50) | 22 (29.3) |
| Mixed race | 1 (3.3) | 3 (4.0) |
| Unknown | — | 3 (4.0) |
| Medical history | ||
| Developmental delay (yes, %) | 3 (10) | 3 (4.0) |
| Autoimmune disease (yes, %) | 2 (6.7) | 5 (6.7) |
| Epilepsy (yes, %) | 4 (13.3) | 8 (10.7) |
| Psychiatric disease (yes, %) | 3 (10) | 11 (14.7) |
| Cancer (yes, %) | 2 (6.7) | 7 (9.3) |
| Clinical characteristics | ||
| Antibody-positive | Pediatric, n = 29 | Adult, n = 59 |
| Subtype, n (%)a | ||
| NMDA | 22 (76) | 20 (34) |
| AMPA | 1 (3) | 2 (4) |
| GAD65b | 4 (14) | 5 (8) |
| VGKC | 1 (3) | 4 (7) |
| TPO | 3 (10) | 9 (16) |
| AchR | — | 3 (5) |
| Amphiphysin | — | 1 (2) |
| ANNA2 | — | 2 (4) |
| CASPR2 | — | 4 (7) |
| LGI1 | — | 8 (14) |
| CRMP5 | — | 2 (4) |
| GABA | — | 1 (2) |
| GFAP | — | 2 (4) |
| Glycine | — | 1 (2) |
| Hu | — | 2 (4) |
| Yo | — | 3 (5) |
| Antibody-negative | Pediatric, n = 1 | Adult, n = 16 |
| Initial presentation | Pediatric | Adult | ||
| Ab-positive, n = 29 | Ab-negative, n = 1 | Ab-positive, n = 59 | Ab-negative, n = 16 | |
| Symptom presentation, n (%) | ||||
| Neuropsychiatric | 25 (86) | 1 (100) | 33 (57) | 7 (44) |
| Seizures | 20 (69) | 1 (100) | 41 (69) | 6 (38) |
| Movement disorder | 13 (45) | 0 (0) | 22 (37) | 6 (38) |
| Encephalopathy | 26 (90) | 0 (0) | 44 (75) | 13 (81) |
| Ataxia | 8 (28) | 0 (0) | 12 (20) | 6 (38) |
| Peripheral neuropathy | 1 (3) | 0 (0) | 7 (12) | 1 (6) |
| Dysautonomia | 2 (7) | 0 (0) | 14 (24) | 4 (25) |
| Other | 11 (38) | 1 (100) | 16 (27) | 4 (25) |
| Duration of symptoms, wk (mean ± SD) | 2.4 ± 2.0 | 4 | 5.6 ± 5.7 | 3.6 ± 3.1 |
| Hospitalization duration, d (median, IQR) | 25.5 (12.5–64.3) | 4.0 | 16.0 (7.0–40.5) | 14.5 (8.0–22.5) |
| ICU admission, n (%) | 12 (41) | 0 (0) | 27 (47) | 5 (31) |
| CSF obtained, n (%) | 28 (97) | 1 (100) | 52 (90) | 16 (100) |
| Pleocytosis | 19 (68) | 0 (0) | 34 (59) | 7 (44) |
| Elevated protein | 2 (7) | 0 (0) | 19 (33) | 2 (12) |
| Oligoclonal bands obtained | 9 (32) | 0 (100) | 23 (39) | 11 (69) |
| Positive (n, %) | 6 (67) | — | 11 (48) | 2 (18) |
| MRI of the brain obtained | 29 (100) | 1 (100) | 53 (90) | 16 (100) |
| Abnormal (n, %) | 11 (38) | 1 (100) | 32 (54) | 15 (94) |
| EEG obtained | 24 (89) | 1 (100) | 47 (80) | 13 (81) |
| Abnormal (n, %) | 22 (92) | 1 (100) | 37 (79) | 10 (77) |
| EMG/NCS obtained | 0 (0) | 0 (0) | 4 (7) | 3 (19) |
| Abnormal (n, %) | — | — | 2 (50) | 3 (100) |
| Tumor found at onset | 2 (7) | 0 (100) | 11 (19) | 2 (12) |
| Time to immunotherapy initiation, wk (median, IQR) | 1.9 (1.1–5.1) | 4.9 | 6.7 (2.7–16.1) | 6.9 (1.6–11.6) |
| Acute Immunotherapies, n (%) | ||||
| IVMP | 27 (93) | 0 (0) | 34 (58) | 13 (81) |
| PO prednisone | 4 (14) | 0 (0) | 25 (42) | 8 (50) |
| IVIG | 27 (93) | 1 (100) | 31 (53) | 8 (50) |
| PLEX | 7 (24) | 0 (0) | 19 (32) | 4 (25) |
| IVMP + IVIG | 25 (86) | 0 (0) | 16 (27) | 6 (38) |
| IVMP + PLEX | 7 (24) | 0 (0) | 19 (32) | 4 (25) |
| IVIG + PLEX | 7 (24) | 0 (0) | 8 (14) | 1 (6) |
| IVMP + IVIG + PLEX | 7 (24) | 0 (0) | 4 (7) | 1 (6) |
| Rituximab | 17 (59) | 0 (0) | 18 (31) | 9 (56) |
| Cyclophosphamide | 3 (10) | 0 (0) | 1 (2) | 1 (6) |
| Bortezomib | 1 (3) | 0 (0) | 0 (0) | 0 (0) |
| Long-term immunotherapy after initial episode, n (%) | 19 (66) | 1 (100) | 31 (58) | 7 (39) |
| IV methylprednisolone | 0 (0) | 0 (0) | 1 (2) | 0 (0) |
| PO prednisone | 4 (14) | 0 (0) | 11 (20) | 1 (7) |
| IVIG | 5 (17) | 0 (0) | 8 (15) | 1 (7) |
| PLEX | 0 (0) | 0 (0) | 0 (0) | 0 (0) |
| Rituximab | 18 (62) | 1 (100)c | 19 (35) | 13 (87) |
| Cyclophosphamide | 2 (7) | 0 (0) | 2 (4) | 0 (0) |
| Mycophenolate mofetil | 0 (0) | 0 (0) | 3 (6) | 0 (0) |
| Duration of rituximab therapy, mo (median, IQR) | 12.8 (12.4–21.4) | NA | 13.2 (12.5–23.7) | 12.6 (12.1–12.8) |
Abbreviations: Ab = antibody; AchR = acetylcholine receptor; AMPA = α-amino-3-hydroxy-5-methyl-4-isoxazolepropionic acid; ANNA2 (Ri):antineuronal nuclear autoantibody type 2; CASPR2 = contactin-associated protein-like 2; CRMP5 = CV2/collapsing response mediator protein; EMG/NCS = electromyogram/nerve conduction study; GABA = gamma-aminobutyric acid; GAD65 = glutamic acid decarboxylase 65; GFAP = glial fibrillary acidic protein; Hu/ANNA1 = antineuronal nuclear autoantibody type 1; ICU = intensive care unit; IVIG = IV immunoglobulins; IVMP = IV methylprednisolone; LGI1 = leucine-rich glioma-inactivated 1; PLEX = plasma exchange; PO = oral; TPO = thyroid peroxidase; VGKC = voltage-gated potassium channel; Yo = Purkinje cell cytoplasmic antibody type 1.
Several patients tested positive for more than one autoantibody.
Of the participants with GAD65 positivity, 1 had positive GAD65 in the CSF, 2 had positivity in both serum and CSF, 1 was concurrent with positive AMPA antibody, 1 was concurrent with CASPR2 and LGI1 antibody, 1 concurrent with NMDA, 1 concurrent with glycine antibody. One patient had positive GAD65 and TPO antibodies who presented with new-onset seizures and encephalopathy with elevated protein in the CSF and abnormal EEG. One patient with only GAD65 serum antibody positivity also had pleocytosis in the CSF and MRI T2 abnormalities.
Patient was started on rituximab outside of institution. Now follow-up data available.