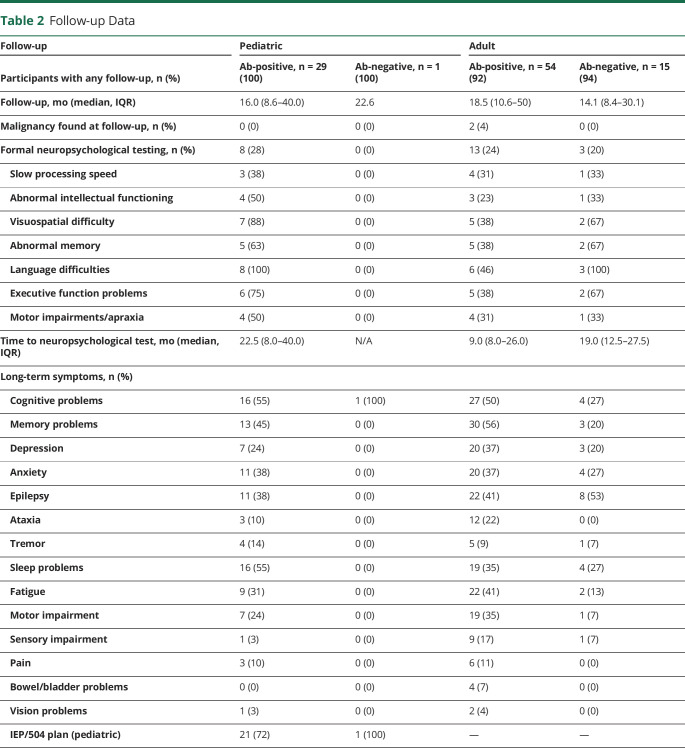
Table 2.
Follow-up Data
| Follow-up | Pediatric | Adult | ||
| Participants with any follow-up, n (%) | Ab-positive, n = 29 (100) | Ab-negative, n = 1 (100) | Ab-positive, n = 54 (92) | Ab-negative, n = 15 (94) |
| Follow-up, mo (median, IQR) | 16.0 (8.6–40.0) | 22.6 | 18.5 (10.6–50) | 14.1 (8.4–30.1) |
| Malignancy found at follow-up, n (%) | 0 (0) | 0 (0) | 2 (4) | 0 (0) |
| Formal neuropsychological testing, n (%) | 8 (28) | 0 (0) | 13 (24) | 3 (20) |
| Slow processing speed | 3 (38) | 0 (0) | 4 (31) | 1 (33) |
| Abnormal intellectual functioning | 4 (50) | 0 (0) | 3 (23) | 1 (33) |
| Visuospatial difficulty | 7 (88) | 0 (0) | 5 (38) | 2 (67) |
| Abnormal memory | 5 (63) | 0 (0) | 5 (38) | 2 (67) |
| Language difficulties | 8 (100) | 0 (0) | 6 (46) | 3 (100) |
| Executive function problems | 6 (75) | 0 (0) | 5 (38) | 2 (67) |
| Motor impairments/apraxia | 4 (50) | 0 (0) | 4 (31) | 1 (33) |
| Time to neuropsychological test, mo (median, IQR) | 22.5 (8.0–40.0) | N/A | 9.0 (8.0–26.0) | 19.0 (12.5–27.5) |
| Long-term symptoms, n (%) | ||||
| Cognitive problems | 16 (55) | 1 (100) | 27 (50) | 4 (27) |
| Memory problems | 13 (45) | 0 (0) | 30 (56) | 3 (20) |
| Depression | 7 (24) | 0 (0) | 20 (37) | 3 (20) |
| Anxiety | 11 (38) | 0 (0) | 20 (37) | 4 (27) |
| Epilepsy | 11 (38) | 0 (0) | 22 (41) | 8 (53) |
| Ataxia | 3 (10) | 0 (0) | 12 (22) | 0 (0) |
| Tremor | 4 (14) | 0 (0) | 5 (9) | 1 (7) |
| Sleep problems | 16 (55) | 0 (0) | 19 (35) | 4 (27) |
| Fatigue | 9 (31) | 0 (0) | 22 (41) | 2 (13) |
| Motor impairment | 7 (24) | 0 (0) | 19 (35) | 1 (7) |
| Sensory impairment | 1 (3) | 0 (0) | 9 (17) | 1 (7) |
| Pain | 3 (10) | 0 (0) | 6 (11) | 0 (0) |
| Bowel/bladder problems | 0 (0) | 0 (0) | 4 (7) | 0 (0) |
| Vision problems | 1 (3) | 0 (0) | 2 (4) | 0 (0) |
| IEP/504 plan (pediatric) | 21 (72) | 1 (100) | — | — |