Abstract
Background
The clinical significance of mitral annular calcification (MAC) in patients undergoing mitral transcatheter edge‐to‐edge repair is not well understood. There is limited evidence regarding the feasibility, durability of repair, and the prognostic value of MAC in this population. We sought to examine the prognostic value of MAC, its severity, and its impact on procedural success and durability of mitral transcatheter edge‐to‐edge repair.
Methods and Results
We reviewed the records of 280 patients with moderate–severe or severe mitral regurgitation who underwent mitral transcatheter edge‐to‐edge repair with MitraClip from March 2014 to March 2022. The primary end point was cumulative survival at 1 year. Independent factors associated with the primary end point were identified using multivariable Cox regression. Among 280 patients included in the final analysis, 249 had none/mild MAC, and 31 had moderate/severe MAC. Median follow‐up was 23.1 months (interquartile range: 11.1–40.4). Procedural success was comparable in the MAC and non‐MAC groups (92.6% versus 91.4%, P=0.79) with similar rates of residual mitral regurgitation ≤2 at 1 year (86.7% versus 93.2%, P=0.55). Moderate/severe MAC was associated with less improvement in New York Heart Association III/IV at 30 days when compared with none/mild MAC (45.8% versus 14.3%, P=0.001). The moderate/severe MAC group had lower cumulative 1‐year survival (56.8% versus 80.0%, hazard ratio [HR], 1.98 [95% CI, 1.27–3.10], P=0.002). Moderate/severe MAC and Society of Thoracic Surgeons predicted risk of mortality for mitral valve repair were independently associated with the primary end point (HR, 2.20 [1.10–4.41], P=0.02; and HR, 1.014 [1.006–1.078], P=0.02, respectively).
Conclusions
Mitral TEER is a safe and feasible intervention in selected patients with significant MAC and associated with similar mitral regurgitation reduction at 1 year compared with patients with none/mild MAC. Patients with moderate/severe MAC had a high 1‐year mortality and less improvement in their symptoms after TEER.
Keywords: mitral annular calcification, mitral regurgitation, TEER
Subject Categories: Catheter-Based Coronary and Valvular Interventions
Nonstandard Abbreviations and Acronyms
- MR
mitral regurgitation
- MV
mitral valve
- TEER
transcatheter edge‐to‐edge repair
Clinical Perspective.
What Is New?
The impact of mitral annular calcification (MAC) on outcomes after mitral transcatheter edge‐to‐edge repair is not well understood. No prior studies have shown increased risk of mortality in patients with moderate/severe MAC after repair with MitraClip.
Society of Thoracic Surgeons predicted risk of mortality for mitral valve repair and MAC severity are of paramount importance in risk stratifying patients before mitral transcatheter edge‐to‐edge repair.
What Are the Clinical Implications?
Although mitral transcatheter edge‐to‐edge repair in carefully selected patients with MAC is safe, feasible, and provides durable MR reduction, higher Society of Thoracic Surgeons predicted risk of mortality for mitral valve repair and MAC portray a worse prognosis for these patients.
Larger multicenter studies with longer‐term follow‐ups are needed to validate our findings in a larger independent cohort.
Mitral annular calcification (MAC) is a degenerative process characterized by deposition of calcium in the mitral valve (MV) annulus, often with extension into the leaflets and chordae. MAC is believed to reflect the manifestation of a systemic inflammatory process. At the level of the MV, MAC can distort the valve geometry, restrict leaflet motion, and impair coaptation, leading to valve dysfunction (regurgitation or stenosis). 1 In the general population, the prevalence of MAC is estimated to range from 8% to 15%. It is even higher in older patients (≈40%), developed countries, and individuals referred for MV surgery (≈24%). 2 , 3 Advanced age, female sex, and other atherosclerosis risk factors such as hypertension, dyslipidemia, diabetes, chronic kidney disease, and hemodialysis are commonly associated with MAC. 4 Clinically, there are established associations between MAC and adverse outcomes including stroke, arrhythmia, heart failure, advanced valve dysfunction, and mortality. 5 , 6 Despite the recent advances in surgical and transcatheter interventions, optimal treatment of patients with MAC and MV dysfunction continues to be a challenge, and MAC remains a strong predictor of postoperative morbidity and mortality. 3 , 7
In patients with high or prohibitive surgical risk with significant mitral regurgitation (MR), mitral transcatheter edge‐to‐edge repair (TEER) has proven to be a beneficial alternative therapy that reduces MR severity, reverses left ventricular remodeling, and improves symptoms. 8 , 9 However, there is limited evidence regarding the feasibility, durability of repair, and the prognostic value of MAC in mitral TEER since these patients were excluded from clinical trials. Two recent studies demonstrated that moderate‐to‐severe MAC was not associated with decreased procedural success. 10 , 11 Similar MR reduction was noted when compared with none‐to‐mild MAC. We sought to investigate the clinical significance of MAC severity and its impact on procedural success, durability of repair, and clinical outcomes in patients who underwent mitral TEER at our institution.
METHODS
The data that support the findings of this study are available from the corresponding author upon reasonable request.
Study Population
We reviewed the records of 294 consecutive patients who had moderate–severe or severe MR and who underwent mitral TEER with MitraClip (Abbott Vascular, Santa Clara, CA) at Houston Methodist Hospital (Houston, TX) from March 2014 to March 2022. As determined by a multidisciplinary heart team based on current guidelines, patients with symptomatic primary MR at prohibitive/high surgical risk and those with secondary MR on maximally tolerated guideline directed medical therapy with feasible anatomy underwent the procedure. Patients who underwent a successful MitraClip procedure constituted our population. Our final cohort was then stratified into 2 main groups: patients with none/mild (N=249) MAC and patients with moderate/severe (N=31) MAC. All study participants gave written informed consent for the use of their medical records for research purposes. The study was approved by the Houston Methodist Institutional Review Board. All study procedures were conducted in accordance with the Declaration of Helsinki.
Echocardiographic Analysis
All patients had preprocedural transthoracic echocardiography and TEE using Philips echocardiography system (i33 instruments; Philips Technology, Amsterdam, Netherlands). American Society of Echocardiography guidelines were used to assess the mechanism and severity of MR as mild (1+), moderate (2+), moderate to severe (3+), and severe (4+). 12 The cause of MR was classified as primary/degenerative, secondary, or mixed based on guidelines. 12 MAC was determined based on the calcium distribution on the annular apparatus: mild: focal noncontiguous calcification limited to <180° of the annular circumference, moderate: continuous calcification 180° to 270°, and severe: continuous calcification ≥270° of total annular circumference (Figure 1). 13
Figure 1. Assessment of mitral annular calcification severity using transesophageal echocardiography.
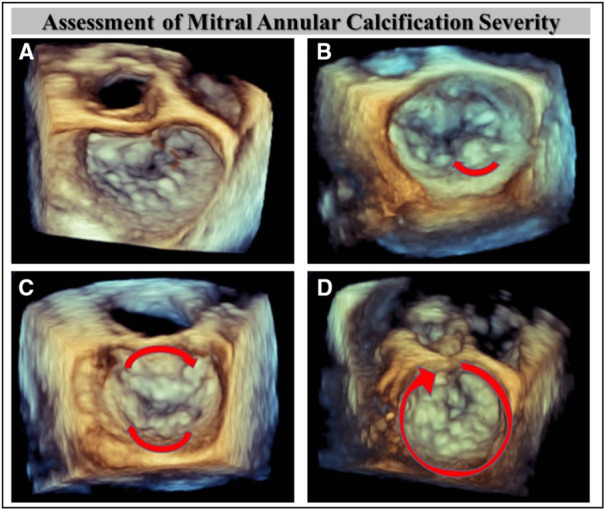
Assessment of calcium burden on the annular apparatus. A, None. B, Mild (focal lesion). C, Moderate (continuous lesion 180°–270°). D, Severe (continuous calcification ≥270°).
Procedural Data and Follow‐Up
The procedure was performed with patients under general anesthesia with transesophageal echocardiography and fluoroscopic guidance. After a transseptal puncture, a 24‐F transseptal sheath was used to measure left atrial pressure (LAP) and V wave at baseline before clip delivery system insertion. LAP and V wave were continuously monitored during the procedure. After the final clip deployment, direct LAP and V wave were measured before withdrawal of the sheath from the LA to the right atrium. Procedural success was defined as postprocedural MR grade less than or equal to moderate. Technical success was defined as the successful deployment of at least 1 clip with no in‐hospital mortality or need for an immediate MV intervention. All patients' medical records were manually reviewed.
The primary end point was cumulative survival at 1 year. Secondary end points studied included degree of MR reduction with TEER, heart failure hospitalizations (HFH) at 1 year, post TEER transmitral mean pressure gradient (TMPG), change in left atrial and left ventricular dimensions, and improvement in New York Heart Association (NYHA) functional class.
Statistical Analysis
Clinical, procedural, and echocardiographic characteristics were collected for all patients before and after the mitral TEER procedure. For categorical variables, frequencies and percentages were used to describe the data. Mean±SD or median with interquartile range were used to summarize continuous variables. The Kolmogorov–Smirnov test was used for assessment of normality for continuous data. A series of Student t tests were utilized to compare the group means for continuous, symmetrical variables. Nonparametric Wilcoxon rank sum tests were used for skewed continuous data. χ2 tests of association or Fisher exact tests (in the event of small counts <5) were used to compare categorical variables. Kaplan–Meier analysis was used to assess survival estimates for the primary end point in the overall population. Comparisons were made using the log rank test.
Cox regression model was used to determine the hazard ratio of MAC on the primary end point. All variables with P <0.10 from univariate analysis in addition to clinically relevant variables chosen a priori and deemed to influence the outcomes of interest were considered for the multivariable Cox regression analysis, and only those with P <0.05 were included in the final model. A 2‐sided P <0.05 was considered statistically significant and all statistical analyses were performed using SPSS version 24.0 (IBM, Armonk, NY).
RESULTS
Baseline Clinical and Echocardiographic Characteristics
Of the 280 patients (mean age 76.7±10.8 years, 43.2% female) included in our final analysis, 148 (52.9%) patients had primary MR, 105 (37.5%) patients had secondary MR, and 27 (9.6%) patients had MR of mixed cause. All patients had MR grade ≥3+, with the vast majority having 4+ (83.3%) MR with NYHA class III/IV present in 218 (80%) patients. Baseline clinical characteristics are listed in Table 1, and baseline echocardiographic variables are listed in Table 2.
Table 1.
Baseline Clinical Characteristics
| Clinical characteristics | Overall N=280 | None/Mild MAC N=249 | Moderate/Severe MAC N=31 | P value |
|---|---|---|---|---|
| Age, y | 76.7 [10.8] | 76.4 [11.0] | 79.0 [8.3] | 0.21 |
| Female | 121 (43.2) | 102 (41.0) | 19 (61.3) | 0.03* |
| Hypertension | 196 (70.0) | 175 (70.3) | 21 (67.7) | 0.77 |
| Atrial fibrillation | 164 (59.0) | 150 (60.7) | 14 (45.2) | 0.09 |
| Smoking | 35 (12.5) | 31 (12.4) | 4 (12.9) | 0.94 |
| Coronary artery disease | 104 (37.1) | 88 (35.3) | 16 (51.6) | 0.07 |
| Frailty | 192 (68.6) | 171 (68.7) | 21 (67.7) | 0.91 |
| Diabetes | 76 (27.1) | 60 (24.1) | 16 (51.6) | 0.001* |
| Prior stroke | 34 (12.1) | 25 (10.0) | 9 (29.0) | 0.006* |
| Dialysis | 19 (6.8) | 10 (4.0) | 9 (29.0) | <0.001* |
| Prior MI | 51 (18.3) | 46 (18.5) | 5 (16.1) | 0.74 |
| Prior pacemaker | 41 (14.6) | 35 (14.1) | 6 (19.4) | 0.42 |
| Prior ICD | 43 (15.4) | 41 (16.5) | 2 (6.5) | 0.19 |
| Prior CABG | 68 (24.3) | 59 (23.7) | 9 (29.0) | 0.51 |
| Prior PCI | 60 (21.4) | 50 (20.1) | 10 (32.3) | 0.11 |
| BMI, kg/m2 | 26.1 [6.0] | 26.1 [6.05] | 26.5 [5.6] | 0.71 |
| BSA, m2 | 1.88 [0.27] | 1.88 [0.22] | 1.81 [0.22] | 0.16 |
| NYHA class | ||||
| I | 8 (2.9) | 8 (3.3) | 0 (0) | 0.63 |
| II | 47 (17.2) | 43 (17.8) | 4 (12.9) | |
| III | 181 (66.3) | 159 (65.7) | 22 (71.0) | |
| IV | 37 (13.6) | 32 (13.2) | 5 (16.1) | |
| STS risk MV repair (%) | 5.3 [5.6] | 4.8 [5.2] | 9.1 [7.2] | 0.006* |
| BNP, pg/mL | 904.0 [1006.2] | 888.0 [1023.6] | 1067.5 [821.6] | 0.54 |
| Hemoglobin, g/dL | 11.5 [2.1] | 11.6 [2.1] | 10.7 [2.5] | 0.04* |
| Creatinine, mg/dL | 1.2 (IQR: 0.9–1.7) | 1.2 (IQR: 0.9–1.5) | 1.5 (IQR: 1.1–2.4) | 0.01* |
Values are expressed as mean [SD], N (%), or median (IQR). BMI indicates body mass index; BNP, B‐type natriuretic peptide; BSA, body surface area; CABG, coronary artery bypass graft; ICD, implantable cardioverter/defibrillator; IQR, interquartile range; MAC, mitral annular calcification; MI, myocardial infarction; MV, mitral valve; NYHA, New York Heart Association; PCI, percutaneous coronary intervention; and STS, Society for Thoracic Surgeons.
P<0.05.
Table 2.
Baseline Echocardiographic Findings
| Echocardiographic characteristics | Overall N=280 | None/Mild MAC N=249 | Moderate/Severe MAC N=31 | P value |
|---|---|---|---|---|
| MR cause | ||||
| Primary | 148 (52.9) | 135 (54.3) | 13 (41.8) | 0.22 |
| Secondary | 105 (37.5) | 93 (37.3) | 12 (38.8) | |
| Mixed | 27 (9.6) | 21 (8.4) | 6 (19.4) | |
| MR severity | ||||
| Moderate–severe | 47 (16.7) | 43 (17.4) | 4 (12.9) | 0.69 |
| Severe | 233 (83.3) | 206 (82.6) | 27 (87.1) | |
| MVA, cm2 | 5.2 [1.7] | 5.4 [1.7] | 4.0 [1.0] | <0.001* |
| TR severity | ||||
| None/trace | 70 (25.0) | 62 (24.9) | 8 (25.8) | 0.40 |
| Mild | 105 (37.5) | 93 (37.3) | 12 (38.7) | |
| Moderate | 76 (27.1) | 66 (26.5) | 10 (32.3) | |
| Severe | 29 (10.4) | 28 (11.2) | 1 (3.2) | |
| PASP, mm Hg | 52.9 [17.74] | 52.4 [17.8] | 56.6 [16.9] | 0.13 |
| RAP, mm Hg | 11.6 [5.9] | 11.5 [5.9] | 12.3 [5.4] | 0.51 |
| LVEF, % | 51.4 [14.7] | 51.2 [14.7] | 52.8 [14.6] | 0.28 |
| LVIDs, cm | 3.7 [1.1] | 3.8 [1.1] | 3.2 [1.0] | 0.013* |
| LVIDd, cm | 5.3 [0.9] | 5.3 [0.9] | 4.9 [0.7] | 0.03* |
| LA volume, mL | 119.0 [54.3] | 121.0 [56.0] | 103.7 [37.1] | 0.02* |
| LAVI, mL/m2 | 63.0 [26.5] | 63.7 [27.2] | 57.9 [20.9] | 0.25 |
Values are expressed as mean [SD] or N (%). LA indicates left atrium; LAVI, left atrium volume index; LVEF, left ventricle ejection fraction; LVIDs/d, left ventricle internal diameter (systole/diastole); MAC, mitral annular calcification; MR, mitral regurgitation; MVA, mitral valve area; PASP, pulmonary artery systolic pressure; RAP, right atrial pressure; and TR, tricuspid regurgitation.
P<0.05.
None/Mild Versus Moderate/Severe MAC
When stratified by MAC severity, 249 (88.9%) patients had none/mild MAC, while 31 (11.1%) had moderate/severe MAC. Compared with the none/mild MAC group, the moderate/severe MAC patients were mostly female (61.3% versus 41.0%, P=0.03), had higher prevalence of diabetes (51.6% versus 35.3%, P=0.001) and prior stroke (29.0% versus 4.0%, P=0.006), and were more likely to be on dialysis (29.0% versus 4.0%, P <0.001). Patients with moderate/severe MAC had higher Society of Thoracic Surgeons (STS) predicted risk of mortality (PROM) for MV repair (9.1%±7.2% versus 4.8%±5.2%, P=0.006). Compared with the patients with none/mild MAC, patients with moderate/severe MAC had smaller: MV area (4.0±1.0 cm2 versus 5.4±1.7 cm2, P<0.001), left ventricle internal diameter/systole (3.2±1.0 cm versus 3.8±1.1 cm, P=0.01), left ventricle internal diameter/diastole (4.9±0.7 cm versus 5.3±0.9 cm, P=0.03), and LA volume (103.7±37.1 mL versus 121.0±5.3 mL, P=0.02).
Except for lower hemoglobin (10.7±2.5 g/dL versus 11.6±2.1 g/dL, P=0.04) and higher creatinine (1.5 mg/dL interquartile range [IQR, 1.1–2.4] versus 1.2 mg/dL [IQR, 0.9–1.5], P=0.01) in the moderate/severe MAC group, there were no significant differences in other baseline clinical and echocardiographic characteristics between the 2 groups.
Procedural and In‐Hospital Outcomes
Procedural details are summarized in Table 3. In the none/mild MAC group, 10 patients had aborted procedures (6 due to high mitral gradient after clip placement, 2 due to inability to reduce MR, and 2 due to inability to grasp the mitral leaflets). In the moderate/severe MAC group, 4 patients had aborted procedures (3 due to high mitral gradient after clip placement, and 1 due to inability to grasp the mitral leaflets).
Table 3.
Procedural Characteristics
| Procedural characteristics | Overall N=280 | None/Mild MAC N=249 | Moderate/Severe MAC N=31 | P value |
|---|---|---|---|---|
| Number of clips | 1.5 [0.6] | 1.5 [0.6] | 1.4 [0.5] | 0.66 |
| 1 Clip | 147 (52.5) | 131 (52.6) | 16 (51.6) | 0.91 |
| 2 Clips | 119 (42.5) | 104 (41.8) | 15 (48.4) | 0.48 |
| >2 Clips | 14 (5.0) | 14 (5.6) | 0 (0) | 0.37 |
| Type of MitraClip | ||||
| Old generation | 120 (42.9) | 103 (41.4) | 17 (54.8) | 0.15 |
| NT Classic | 15 (5.4) | 11 (4.4) | 4 (12.9) | 0.07 |
| NTR | 12 (4.3) | 12 (4.8) | 0 (0) | 0.37 |
| XTR | 31 (11.1) | 29 (11.6) | 2 (6.5) | 0.54 |
| NTW | 55 (19.6) | 53 (21.3) | 2 (6.5) | 0.05 |
| XTW | 36 (12.9) | 29 (11.6) | 7 (22.6) | 0.09 |
| Fluoroscopy time, min | 22.8 [17.3] | 23.2 [17.9] | 19.9 [10.0] | 0.33 |
| Left atrial pressure, mm Hg | 19.8 [7.7] | 19.7 [7.9] | 20.9 [6.2] | 0.43 |
| V wave, mm Hg | 34.5 [17.1] | 33.7 [17.3] | 40.5 [14.8] | 0.04* |
| Post‐clip | ||||
| MR severity | ||||
| None/trace | 52 (18.6) | 48 (19.3) | 4 (12.9) | 0.30 |
| Mild | 181 (64.7) | 157 (63.0) | 24 (77.4) | |
| Moderate | 38 (13.6) | 35 (14.1) | 3 (9.7) | |
| Moderate–severe | 6 (2.1) | 6 (2.4) | 0 (0) | |
| Severe | 3 (1.1) | 3 (1.2) | 0 (0) | |
| LAP, mm Hg | 15.1 [5.9] | 15.09 [6.0] | 15.48 [5.0] | 0.73 |
| V wave, mm Hg | 21.8 [9.5] | 21.72 [9.7] | 22.6 [8.2] | 0.63 |
| Difference pre‐ and postclip | ||||
| MR reduction, grade | 2.7 [0.8] | 2.7 [0.8] | 2.8 [0.6] | 0.38 |
| LAP reduction, mm Hg | 4.9 [6.4] | 4.8 [6.5] | 5.4 [5.4] | 0.64 |
| V wave reduction, mm Hg | 13.2 [15.0] | 12.7 [15.1] | 17.5 [13.3] | 0.10 |
Values are expressed as mean [SD] or N (%). LAP indicates left atrial pressure; MAC, mitral annular calcification; and MR, mitral regurgitation.
P<0.05.
Among patients with successful mitral TEER, the moderate/severe MAC group had higher left atrial V wave pressure compared with patients with none/mild MAC (40.5±14.8 mm Hg versus 33.7±17.3 mm Hg, P=0.04). Mean left atrial pressure was comparable at baseline between the 2 groups. Total number of clips deployed averaged 1.5±0.6 per patient, with no differences in the number and generation of MitraClip used between the 2 groups. None/mild and moderate/severe MAC groups had comparable technical (94.9% versus 88.5%, P=0.12) and procedural success (92.6% versus 91.4%, P=0.79) (Figure 2). Residual MR grade ≤mild was achieved in 83.3% of patients at the end of the procedure (Figure 3), with no difference between the 2 groups. Residual MR >moderate was present in 3.3% of patients (3.6% in none/mild MAC group versus 0% in moderate/severe MAC group). Overall, a 2.7 grade reduction in MR severity was achieved (2.7 grade in none/mild MAC group versus 2.8 grade reduction in moderate/severe MAC group, P=0.38). The degree of reduction in mean LA pressure and V wave pressure was similar in both groups. The median length of stay was 1 day (IQR, 1–3). There was no in‐hospital mortality in the moderate/severe MAC group, while 3 patients (1.2%) had in‐hospital mortality in the none/mild MAC group. At hospital discharge, patients with moderate/severe MAC had higher TMPG (5.5±2.1 mm Hg versus 4.0±1.8 mm Hg, P<0.001). The proportion of patients with gradient >5 mm Hg was higher in the moderate/severe MAC group (48.3% versus 20.4%, P<0.001).
Figure 2. Secondary outcomes after TEER according to MAC severity.
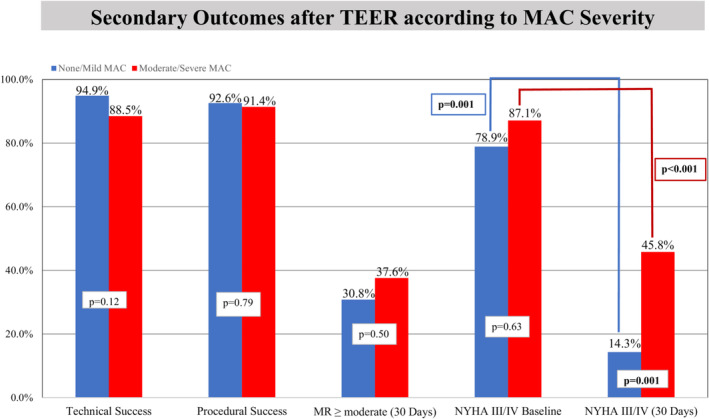
Compared with moderate/severe MAC patients, none/mild MAC patients had comparable technical and procedural success. Both groups had similar MR reduction at 1 month and NYHA class III/IV at baseline. Both groups had significant improvement in NYHA class at 1 month, but the improvement was more pronounced in the none/mild MAC group (14.3% vs 45.8%, P=0.001). MAC indicates mitral annular calcification; MR, mitral regurgitation; NYHA, New York Heart Association; and TEER, transcatheter edge‐to‐edge repair.
Figure 3. Mitral regurgitation severity before and after MitraClip.
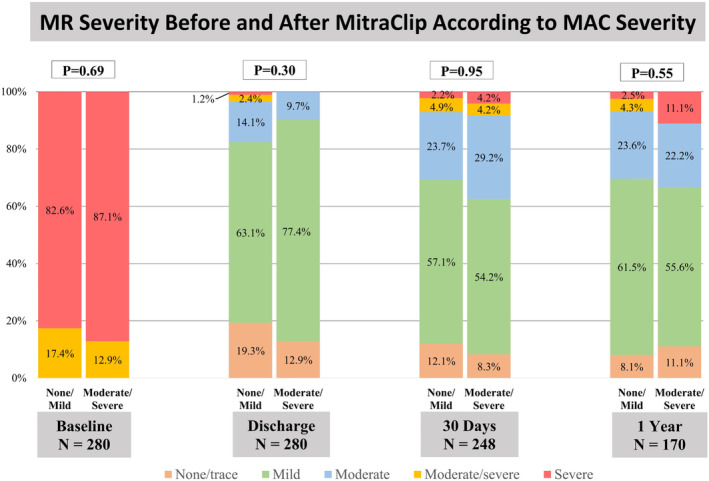
There was a significant reduction in MR severity in the non/mild and moderate/severe MAC groups after MitraClip implantation with ≤moderate MR in 96.4% vs 100% at discharge, 92.9% vs 91.6% at 30 days, and 93.2% vs 88.9% at 1 year (all P<0.001). MAC indicates mitral annular calcification; and MR, mitral regurgitation.
Thirty‐Day and 1‐Year Outcomes
These are presented in Table 4. At 30 days, 10 patients (3.6%) died, and 6 (2.1%) had HFH. Mortality and HFH were not different between the 2 groups. Both groups had comparable residual MR severity (≤moderate in 92.9% none/mild versus 91.6% moderate/severe MAC, P=0.95) (Figure 2). Patients in the moderate/severe MAC were more symptomatic with higher prevalence of NYHA class III/IV (45.8% versus 14.3%, P=0.001) in addition to having a higher TMPG (5.6±2.7 mm Hg versus 4.3±2.2 mm Hg, P=0.01) compared with the none/mild MAC group (Figure 2).
Table 4.
Outcomes
| Outcomes | Overall N=280 | None/Mild MAC N=249 | Moderate/Severe MAC N=31 | P value |
|---|---|---|---|---|
| In‐hospital outcomes | ||||
| Length of stay, d | 2 (IQR:1–3) | 2 (IQR:1–3) | 1 (IQR:1–5.5) | 0.28 |
| In‐hospital mortality | 3 (1.0) | 3 (1.2) | 0 (0) | 0.99 |
| Transmitral mean gradient, mm Hg | 4.1 [1.94] | 4.0 [1.8] | 5.5 [2.1] | <0.001* |
| Transmitral mean gradient >5 mm Hg | 66 (23.5) | 51 (20.4) | 15 (48.3) | <0.001* |
| N=248 | N=224 | N=24 | ||
|---|---|---|---|---|
| 30‐d outcomes | ||||
| Mortality | 10 (3.6) | 8 (3.2) | 2 (6.5) | 0.30 |
| Heart failure hospitalization | 6 (2.1) | 5 (2.0) | 1 (3.2) | 0.50 |
| NYHA class III or IV | 41 (17.5) | 30 (14.3) | 11 (45.8) | 0.001* |
| LA volume, mL | 122.0 [50.1] | 122.7 [51.4] | 116.0 [37.7] | 0.54 |
| LAVI, mL/m2 | 68.4 [26.0] | 68.6 [26.4] | 66.7 [22.6] | 0.74 |
| PASP, mm Hg | 47.8 [14.3] | 47.6 [14.2] | 49.6 [15.2] | 0.70 |
| LVIDs, cm | 3.7 [1.1] | 3.8 [1.1] | 3.4 [1.1] | 0.13 |
| LVIDd, cm | 5.1 [0.8] | 5.2 [0.8] | 4.8 [0.7] | 0.05 |
| MR severity | ||||
| None/trace | 29 (11.7) | 27 (12.1) | 2 (8.3) | 0.95 |
| Mild | 141 (56.9) | 128 (57.2) | 13 (54.1) | |
| Moderate | 60 (24.2) | 53 (23.7) | 7 (29.2) | |
| Moderate–severe | 12 (4.8) | 11 (4.9) | 1 (4.2) | |
| Severe | 6 (2.4) | 5 (2.2) | 1 (4.2) | |
| Transmitral mean gradient, mm Hg | 4.4 [2.3] | 4.3 [2.2] | 5.6 [2.7] | 0.01* |
| TR severity | ||||
| ≥Moderate | 70 (28.9) | 63 (28.8) | 7 (29.1) | 0.84 |
| N=176 | N=161 | N=15 | ||
|---|---|---|---|---|
| 1‐y Outcomes | ||||
| Mortality | 62 (22.1) | 49 (19.7) | 13 (41.9) | 0.005* |
| Heart failure hospitalization | 32 (11.4) | 29 (11.6) | 3 (9.7) | 1.00 |
| LA volume, mL | 120.9 [46.5] | 121.6 [47.3] | 112.1 [36.1] | 0.49 |
| LAVI | 65.8 [25.8] | 65.8 [25.7] | 65.8 [27.3] | 0.99 |
| LVIDs, cm | 3.6 [1.1] | 3.6 [1.1] | 3.2 [0.6] | 0.03* |
| LVIDd, cm | 5.0 [0.9] | 5.1 [0.9] | 4.6 [0.6] | 0.04* |
| MR severity | ||||
| None/trace | 14 (8.0) | 13 (8.1) | 1 (6.7) | 0.55 |
| Mild | 106 (60.2) | 99 (51.4) | 7 (46.7) | |
| Moderate | 43 (24.4) | 38 (23.6) | 5 (33.3) | |
| Moderate–severe | 9 (5.1) | 7 (4.3) | 2 (13.3) | |
| Severe | 4 (2.3) | 4 (2.5) | 0 (0) | |
| Transmitral mean gradient, mm Hg | 4.28 [2.23] | 4.2 [2.2] | 5.0 [2.2] | 0.17 |
| TR severity | ||||
| ≥Moderate | 42 (26.1) | 39 (26.4) | 3 (23.1) | 0.25 |
| N=280 | N=249 | N=31 | ||
|---|---|---|---|---|
| Last follow‐up | ||||
| Median follow‐up, mo | 23.1 (IQR:11.1–40.4) | 23.1 (IQR: 11.1–40.4) | 24.0 (IQR: 11.9–42) | 0.28 |
| Mortality | 140 (50.0) | 116 (46.6) | 24 (77.4) | 0.001* |
| Heart failure hospitalization | 58 (20.7) | 54 (21.7) | 4 (12.9) | 0.25 |
| MV reintervention | 9 (3.2) | 7 (2.8) | 2 (6.5) | 0.26 |
Values are expressed as mean [SD], N (%), or median (IQR). IQR indicates interquartile range; LAVI, left atrium volume index; LVIDd, left ventricle internal diameter end diastole; LVIDs, left ventricle internal diameter end systole; MAC, mitral annular calcification; MR, mitral regurgitation; MV, mitral valve; NYHA, New York Heart Association; PASP, pulmonary artery systolic pressure; and TR, tricuspid regurgitation.
P<0.05.
At 1 year, patients with moderate/severe MAC had a significantly lower cumulative survival rate when compared with the patients with none/mild MAC (56.8% versus 80.0%, hazard ratio [HR], 1.98 [1.27–3.10], P=0.002). HFH rates were similar between the 2 groups. Both groups had comparable residual ≤moderate MR severity (93.2% versus 88.9%, P=0.55) with the moderate/severe MAC group having smaller left ventricle internal diameter/systole (3.2±0.6 cm versus 3.6±1.1 cm, P=0.03) and smaller left ventricle internal diameter/diastole (4.6±0.6 cm versus 5.1±0.9 cm, P=0.04). MV reintervention rates were comparable between the 2 groups (6.5% versus 2.8%, P=0.26).
At the last follow‐up, the moderate/severe MAC group continued to have a lower cumulative survival rate when compared with the none/mild MAC group (44.5% versus 57.3%; HR, 1.98 [1.27–3.10], P=0.002) with comparable absolute HFH rates (21.7% versus 12.9%, P=0.25) (Figure 4; Table 4).
Figure 4. Survival analysis according MAC severity.
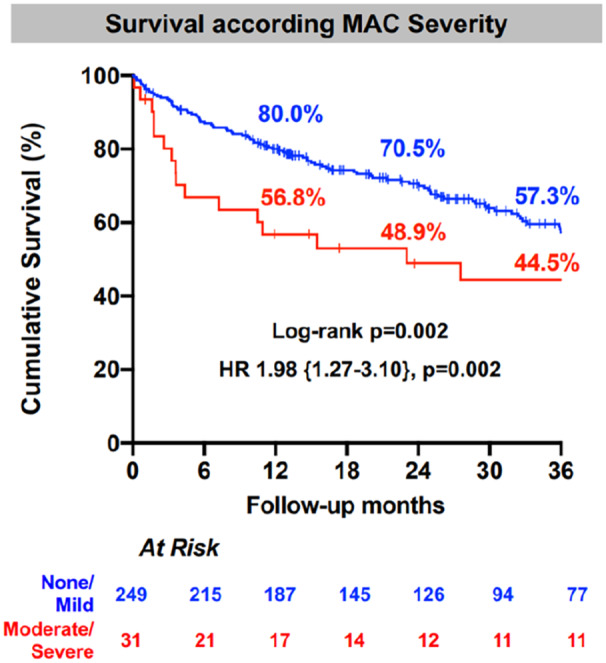
Compared with the moderate/severe MAC group, cumulative survival was higher in the none/mild MAC group at 1, 2, and 3 years of follow‐up. HR indicates hazards ratio; and MAC, mitral annular calcification.
Factors Independently Associated With 1‐Year Mortality
One‐year mortality after mitral TEER was associated with lower baseline ejection fraction, higher postprocedural LAP, higher STS PROM for MV repair, TMPG >5 mm Hg, and moderate/severe MAC. After multivariable adjustment, moderate/severe MAC, and STS PROM for MV repair continued to be independently associated with 1‐year mortality (HR, 2.21 [1.10–4.42], P=0.025, and HR, 1.014 [1.006–1.078], P=0.020, respectively) (Figure 5).
Figure 5. Factors independently associated with 1‐year mortality after mitral TEER.
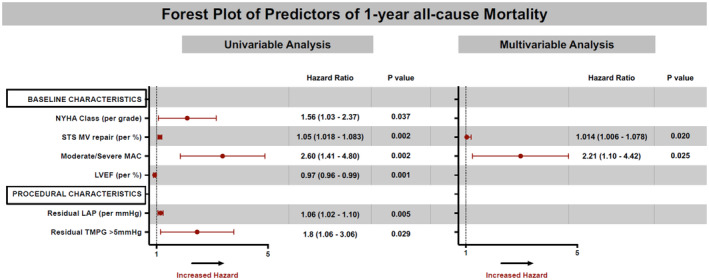
Forest plot showing univariable and multivariable associations of 1‐year mortality after mitral TEER. MAC severity was independently associated with 1‐year mortality after TEER [HR=2.20 [1.10–4.41], P=0.02]. HR indicates hazard ratio; LAP, left atrial pressure; LVEF, left ventricular ejection fraction; MAC, mitral annular calcification; NYHA, New York Heart Association; STS MV repair, Society of Thoracic Surgeons' risk of mortality for mitral valve repair; TEER, transcatheter edge‐to‐edge repair; and TMPG, transmitral mean pressure gradient.
DISCUSSION
The main findings of our study are as follows (Figure 6): First, patients with severe MR and moderate/severe MAC have a higher burden of comorbidities and are at higher surgical risk compared with those with none/mild MAC. Second, mitral TEER is a safe and feasible intervention in select patients with significant MAC, with similar degree of procedural success and MR reduction in those with moderate/severe MAC compared with none/mild MAC. Third, patients with moderate/severe MAC had smaller left chamber dimensions and higher postprocedural TMPG. Fourth, TEER in patients with moderate/severe MAC was associated with durable MR reduction and similar rates of HFH at 30‐day and 1‐year follow‐up. Fifth, patients with moderate/severe MAC have a significantly higher 1‐year mortality compared with patients with none/mild MAC. Finally, multivariate analysis revealed that moderate/severe MAC and STS PROM were independently associated with 1‐year mortality after TEER.
Figure 6. TEER outcomes according to mitral annular calcification severity.
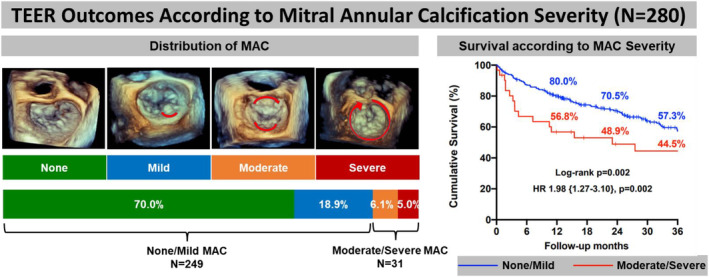
Distribution of MAC in the population: none 70%, mild 18.9%, moderate 6.1%, and severe 5.0%. Compared with the none/mild MAC group, patients with moderate/severe MAC had lower overall cumulative survival at 3 years (44.5% vs 57.3%, log‐rank P=0.002), with an incremental risk of mortality (HR, 1.98 [1.27–3.10], P=0.002). HR indicates hazards ratio; MAC, mitral annular calcification; and TEER, transcatheter edge‐to‐edge repair.
Despite recent advances in surgical techniques, MAC remains a challenge during MV surgery and is associated with adverse outcomes. 14 , 15 In patients with high surgical risk, mitral TEER has proven to be an attractive alternative. 8 , 9 The presence of significant MAC is often associated with older age, diabetes, dialysis, history of stroke, and other medical comorbidities. Patients with MAC commonly have high STS PROM scores and get declined for surgery. Such patients have challenging anatomy and have been considered unsuitable for TEER and have been excluded from clinical trials evaluating TEER devices. Therefore, studying the impact of MAC on outcomes after mitral TEER is needed in a real‐world setting.
To date, only 2 studies have evaluated TEER in patients with MAC and demonstrated that mitral TEER is feasible in patients with significant MAC with similar procedural success and MR reduction rates when compared with none/mild MAC. 10 , 11 However, 1 study showed that moderate‐to‐severe MAC was not associated with all‐cause mortality, 10 while the other demonstrated a trend towards higher overall mortality after TEER. 11
In our cohort, patients with moderate‐to‐severe MAC constituted a high surgical risk population with an average age of 79 years, high prevalence of diabetes, stroke, dialysis, and a high STS PROM of 9.1% for MV repair. This observation stands in contrast with the mean STS score of 4% in the study by Fernandez‐Peregrina et al. 11 The moderate/severe MAC group had smaller LV dimensions and MV area compared with the none/mild MAC group despite similar MR severity. Smaller LV dimensions could be related to the pathophysiological processes that MAC shares with other diseases leading to left ventricular hypertrophy and diastolic dysfunction such as chronic kidney disease, aortic stenosis, and hypertension. 4 , 16 Lower MV area can be attributed to mixed MAC‐related valvular dysfunction. A surgical series showed that concomitant mitral stenosis and regurgitation was present in ≈31% of patients with MAC‐related valve dysfunction. 17 It is conceivable from a physiological standpoint that mitral stenosis and lower baseline MV area are the cause of increased TMPG seen at discharge and at 1 month in the moderate‐to‐severe MAC group when compared with the none/mild MAC group despite a similar number of clips deployed and similar baseline and postprocedural LAP.
Procedural and technical success was comparable in the moderate/severe MAC and none/mild MAC groups (88.5% versus 94.9%, P=0.12 and 91.4% versus 92.6%, P=0.79, respectively). Rates and causes of aborted procedures were similar between the 2 groups. There was no in‐hospital mortality or conversion to mitral surgery in the moderate/severe MAC group. At the last follow‐up, the need for subsequent MV re‐intervention remained low in the moderate/severe MAC group (6.5%) and was comparable to the none/mild MAC group (2.8%, P=0.26). These results are concordant with prior studies, 10 , 11 making mitral TEER an attractive alternative to surgical edge‐to‐edge mitral repair, in which patients with annular calcification had higher reintervention rates (23%) than those patients without (5%). 18 , 19 However, it is important to note that a carefully selected group of patients with significant MAC underwent the procedure. This should be taken into consideration when analyzing the feasibility of mitral TEER in this population.
In terms of durability of repair, mitral TEER resulted in sustained clinical and echocardiographic improvement. Clinically, NYHA functional class improved in both none/mild MAC (NYHA class III/IV 79% at baseline to 14.3% at 1 month, P <0.001) and MAC groups (87% at baseline to 45.8% at 1 month, P=0.001) but remained higher in the moderate‐to‐severe MAC group at 1 month (14.3% versus 45.8%, P=0.001). This can be explained by the fact that the MAC cohort had smaller LV dimensions, higher baseline V wave, and higher postprocedural TMPG, which implies diastolic dysfunction and higher residual pulmonary hypertension. Echocardiographically, both groups had significant postprocedural reduction of MR that was sustained at 1 month (residual MR ≤2 in 92.9% of non‐MAC and 91.6% of MAC patients) and 1 year (residual MR ≤2 in 93.2% of non‐MAC and 86.7% of MAC patients). These findings translated to comparable HFH in both groups in the year following the intervention (11.6% in no MAC versus 9.7% in MAC, P=1.00). These findings are promising regarding the safety of mitral TEER and its ability to reduce HFH in select patients with severe MR, significant MAC, and high surgical risk.
Factors Independently Associated With 1‐Year Mortality
In our study cohort, despite successful mitral TEER, patients with moderate/severe MAC had a very high 1‐year mortality when compared with the none/mild MAC group. This likely reflects an underlying systemic process with accelerated atherosclerosis coupled with a high burden of comorbidities in patients with moderate/severe MAC. Data from the STS transcatheter valve therapies Registry show a high, 22% 30‐day mortality in patients undergoing valve‐in‐MAC transcatheter MV replacement. 20 Similarly, data from the transcatheter mitral valve replacement in the MAC Global Registry including 116 patients undergoing transcatheter mitral valve replacement showed a 53.7% 1‐year mortality in such patients. 21 Our findings are concordant with these registry data showing high mortality despite successful MV interventions in patients with significant MAC. Additionally, baseline STS PROM for MV repair and moderate/severe MAC were independently associated with 1‐year mortality after TEER and the mortality difference was sustained until the last follow‐up. Our findings are consistent with a recent meta‐analysis where the presence MAC was an independent predictor of mortality and major adverse cardiovascular events. 22
The prognostic impact of STS PROM for predicting long‐term outcomes in mitral TEER has been controversial. Some studies showed that STS score had low sensitivity in predicting long‐term mortality, 23 , 24 while other studies showed that higher STS PROM was linked with higher long‐term all cause‐mortality. 25 In one of the earlier studies about feasibility of mitral TEER in MAC patients, STS PROM was not an independent predictor of outcomes. 10 The cohort studied had comparable individual risk factors between MAC and non‐MAC patients, unlike our population where MAC patients had more risk factors at baseline (higher prevalence of stroke, dialysis, and diabetes). This led to a higher STS score for our MAC population, and consequently, STS score being independently associated with 1‐year mortality. Therefore, our findings emphasize the importance of integrating STS PROM for MV repair and MAC severity in risk‐stratifying patients and guiding decisions for mitral TEER procedures and perhaps other mitral interventions in those who are not candidates for TEER.
Study Limitations
Our study has several limitations. First, the retrospective nature of this study at a single institution has inherent limitations and biases, including time bias because different TEER device generations were included. Second, MAC was qualitative and based on echocardiography, a very small number of patients had prior computed tomography. Among those, MAC grading on echocardiography and computed tomography was concordant in 11/12 (91.6%) patients, implying acceptable assessment of MAC severity. Third, the MAC cohort was a carefully selected population that was thought to have acceptable, favorable, and nonprohibitive anatomy for mitral TEER. Fourth, the limited number of patients in the moderate/severe MAC group constituted a limiting factor that might have affected the overall follow‐up and carrying of meaningful analysis.
CONCLUSIONs
MAC reflects an underlying systemic process in patients with a high burden of comorbidities. In select patients with severe MR, moderate/severe MAC, and high risk for MV surgery, mitral TEER is a safe and a feasible intervention associated with similar procedural success, MR reduction, and HFH at 1 year compared with patients with none/mild MAC. However, patients with significant MAC had less improvement in their symptoms and high 1‐year mortality. Risk prediction models are needed to identify MAC patients who benefit most with TEER.
Sources of Funding
None.
Disclosures
Dr Reardon is a consultant for Medtronic, Boston Scientific, Abbott, and W L Gore & Associates. Dr Atkins is a consultant for W L Gore & Associates. Dr Kleiman is a local principal investigator in trials sponsored by Boston Scientific, Medtronic, Abbott, and Edwards Lifesciences. Dr Goel is a consultant for Medtronic, W L Gore & Associates, and on the Speakers Bureau for Abbott Structural Heart. The remaining authors have no disclosures to report.
This study was presented as a Moderated Abstract at the Cardiovascular Research Technologies Annual Meeting, February 25–28, 2023.
This manuscript was sent to Amgad Mentias, MD, Associate Editor, for review by expert referees, editorial decision, and final disposition.
For Sources of Funding and Disclosures, see page 11.
References
- 1. Silbiger JJ. Anatomy, mechanics, and pathophysiology of the mitral annulus. Am Heart J. 2012;164:163–176. doi: 10.1016/j.ahj.2012.05.014 [DOI] [PubMed] [Google Scholar]
- 2. Barasch E, Gottdiener JS, Larsen EK, Chaves PH, Newman AB, Manolio TA. Clinical significance of calcification of the fibrous skeleton of the heart and aortosclerosis in community dwelling elderly. The Cardiovascular Health Study (CHS). Am Heart J. 2006;151:39–47. doi: 10.1016/j.ahj.2005.03.052 [DOI] [PubMed] [Google Scholar]
- 3. Fusini L, Ghulam Ali S, Tamborini G, Muratori M, Gripari P, Maffessanti F, Celeste F, Guglielmo M, Cefalù C, Alamanni F, et al. Prevalence of calcification of the mitral valve annulus in patients undergoing surgical repair of mitral valve prolapse. Am J Cardiol. 2014;113:1867–1873. doi: 10.1016/j.amjcard.2014.03.013 [DOI] [PubMed] [Google Scholar]
- 4. Elmariah S, Budoff MJ, Delaney JA, Hamirani Y, Eng J, Fuster V, Kronmal RA, Halperin JL, O'Brien KD. Risk factors associated with the incidence and progression of mitral annulus calcification: the multi‐ethnic study of atherosclerosis. Am Heart J. 2013;166:904–912. doi: 10.1016/j.ahj.2013.08.015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Bertrand PB, Churchill TW, Yucel E, Namasivayam M, Bernard S, Nagata Y, He W, Andrews CT, Picard MH, Weyman AE, et al. Prognostic importance of the transmitral pressure gradient in mitral annular calcification with associated mitral valve dysfunction. Eur Heart J. 2020;41:4321–4328. doi: 10.1093/eurheartj/ehaa819 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Benjamin EJ, Plehn JF, D'Agostino RB, Belanger AJ, Comai K, Fuller DL, Wolf PA, Levy D. Mitral annular calcification and the risk of stroke in an elderly cohort. N Engl J Med. 1992;327:374–379. doi: 10.1056/NEJM199208063270602 [DOI] [PubMed] [Google Scholar]
- 7. Guerrero M, Dvir D, Himbert D, Urena M, Eleid M, Wang DD, Greenbaum A, Mahadevan VS, Holzhey D, O'Hair D, et al. Transcatheter mitral valve replacement in native mitral valve disease with severe mitral annular calcification: results from the first multicenter global registry. JACC Cardiovasc Interv. 2016;9:1361–1371. doi: 10.1016/j.jcin.2016.04.022 [DOI] [PubMed] [Google Scholar]
- 8. Siegel RJ, Biner S, Rafique AM, Rinaldi M, Lim S, Fail P, Hermiller J, Smalling R, Whitlow PL, Herrmann HC, et al. The acute hemodynamic effects of MitraClip therapy. J Am Coll Cardiol. 2011;57:1658–1665. doi: 10.1016/j.jacc.2010.11.043 [DOI] [PubMed] [Google Scholar]
- 9. Glower DD, Kar S, Trento A, Lim DS, Bajwa T, Quesada R, Whitlow PL, Rinaldi MJ, Grayburn P, Mack MJ, et al. Percutaneous mitral valve repair for mitral regurgitation in high‐risk patients: results of the EVEREST II study. J Am Coll Cardiol. 2014;64:172–181. doi: 10.1016/j.jacc.2013.12.062 [DOI] [PubMed] [Google Scholar]
- 10. Cheng R, Tat E, Siegel RJ, Arsanjani R, Hussaini A, Makar M, Mizutani Y, Trento A, Kar S. Mitral annular calcification is not associated with decreased procedural success, durability of repair, or left ventricular remodeling in percutaneous edge‐to‐edge repair of mitral regurgitation. EuroIntervention. 2016;12:1176–1184. doi: 10.4244/EIJV12I9A191 [DOI] [PubMed] [Google Scholar]
- 11. Fernández‐Peregrina E, Pascual I, Freixa X, Tirado‐Conte G, Estévez‐Loureiro R, Carrasco‐Chinchilla F, Benito‐González T, Asmarats L, Sanchís L, Jiménez‐Quevedo P, et al. Transcatheter edge‐to‐edge mitral valve repair in patients with mitral annulus calcification. EuroIntervention. 2022;17:1300–1309. doi: 10.4244/EIJ-D-21-00205 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Zoghbi WA, Enriquez‐Sarano M, Foster E, Grayburn PA, Kraft CD, Levine RA, Nihoyannopoulos P, Otto CM, Quinones MA, Rakowski H, et al; American Society of Echocardiography. Recommendations for evaluation of the severity of native valvular regurgitation with two‐dimensional and Doppler echocardiography. J Am Soc Echocardiogr. 2003;16:777–802. doi: 10.1016/S0894-7317(03)00335-3 [DOI] [PubMed] [Google Scholar]
- 13. Eleid MF, Foley TA, Said SM, Pislaru SV, Rihal CS. Severe mitral annular calcification: multimodality imaging for therapeutic strategies and interventions. JACC Cardiovasc Imaging. 2016;9:1318–1337. doi: 10.1016/j.jcmg.2016.09.001 [DOI] [PubMed] [Google Scholar]
- 14. Tomšič A, Hiemstra YL, van Brakel TJ, Versteegh MIM, Marsan NA, Klautz RJM, Palmen M. Outcomes of valve repair for degenerative disease in patients with mitral annular calcification. Ann Thorac Surg. 2019;107:1195–1201. doi: 10.1016/j.athoracsur.2018.08.017 [DOI] [PubMed] [Google Scholar]
- 15. Saran N, Greason KL, Schaff HV, Cicek SM, Daly RC, Maltais S, Stulak JM, Pochettino A, King KS, Dearani JA, et al. Does mitral valve calcium in patients undergoing mitral valve replacement portend worse survival? Ann Thorac Surg. 2019;107:444–452. doi: 10.1016/j.athoracsur.2018.07.098 [DOI] [PubMed] [Google Scholar]
- 16. Iwataki M, Takeuchi M, Otani K, Kuwaki H, Yoshitani H, Abe H, Lang RM, Levine RA, Otsuji Y. Calcific extension towards the mitral valve causes non‐rheumatic mitral stenosis in degenerative aortic stenosis: real‐time 3D transesophageal echocardiography study. Open Heart. 2014;1:e000136. doi: 10.1136/openhrt-2014-000136 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Uchimuro T, Fukui T, Shimizu A, Takanashi S. Mitral valve surgery in patients with severe mitral annular calcification. Ann Thorac Surg. 2016;101:889–895. doi: 10.1016/j.athoracsur.2015.08.071 [DOI] [PubMed] [Google Scholar]
- 18. De Bonis M, Lapenna E, Maisano F, Barili F, La Canna G, Buzzatti N, Pappalardo F, Calabrese M, Nisi T, Alfieri O. Long term results (≤18 years) of the edge‐to‐edge mitral valve repair without annuloplasty in degenerative mitral regurgitation: implications for the percutaneous approach. Circulation. 2014;130:S19–S24. doi: 10.1161/CIRCULATIONAHA.113.007885 [DOI] [PubMed] [Google Scholar]
- 19. Maisano F, Caldarola A, Blasio A, De Bonis M, La Canna G, Alfieri O. Midterm results of edge‐to‐edge mitral valve repair without annuloplasty. J Thorac Cardiovasc Surg. 2003;126:1987–1997. doi: 10.1016/S0022-5223(03)01291-1 [DOI] [PubMed] [Google Scholar]
- 20. Guerrero M, Vemulapalli S, Xiang Q, Wang DD, Eleid M, Cabalka AK, Sandhu G, Salinger M, Russell H, Greenbaum A, et al. Thirty‐day outcomes of transcatheter mitral valve replacement for degenerated mitral bioprostheses (valve‐in‐valve), failed surgical rings (valve‐in‐ring), and native valve with severe mitral annular calcification (valve‐in‐mitral annular calcification) in the United States: data from the Society of Thoracic Surgeons/American College of Cardiology/Transcatheter Valve Therapy Registry. Circ Cardiovasc Interv. 2020;13:e008425. doi: 10.1161/CIRCINTERVENTIONS.119.008425 [DOI] [PubMed] [Google Scholar]
- 21. Guerrero M, Urena M, Himbert D, Wang DD, Eleid M, Kodali S, George I, Chakravarty T, Mathur M, Holzhey D, et al. 1‐year outcomes of transcatheter mitral valve replacement in patients with severe mitral annular calcification. J Am Coll Cardiol. 2018;71:1841–1853. doi: 10.1016/j.jacc.2018.02.054 [DOI] [PubMed] [Google Scholar]
- 22. Wang TKM, Griffin BP, Xu B, Rodriguez LL, Popovic ZB, Gillinov MA, Pettersson GB, Desai MY. Relationships between mitral annular calcification and cardiovascular events: a meta‐analysis. Echocardiography. 2020;37:1723–1731. doi: 10.1111/echo.14861 [DOI] [PubMed] [Google Scholar]
- 23. Adamo M, Capodanno D, Cannata S, Giannini C, Laudisa ML, Barbanti M, Curello S, Immè S, Maffeo D, Grasso C, et al; GRASP‐IT Investigators. Comparison of three contemporary surgical scores for predicting all‐cause mortality of patients undergoing percutaneous mitral valve repair with the MitraClip system (from the multicenter GRASP‐IT registry). Am J Cardiol. 2015;115:107–112. doi: 10.1016/j.amjcard.2014.09.051 [DOI] [PubMed] [Google Scholar]
- 24. Buccheri S, Capodanno D, Barbanti M, Popolo Rubbio A, Di Salvo ME, Scandura S, Mangiafico S, Ronsivalle G, Chiarandà M, Capranzano P, et al. A risk model for prediction of 1‐year mortality in patients undergoing MitraClip implantation. Am J Cardiol. 2017;119:1443–1449. doi: 10.1016/j.amjcard.2017.01.024 [DOI] [PubMed] [Google Scholar]
- 25. Triantafyllis AS, Kortlandt F, Bakker AL, Swaans MJ, Eefting FD, van der Heyden JA, Post MC, Rensing BW. Long‐term survival and preprocedural predictors of mortality in high surgical risk patients undergoing percutaneous mitral valve repair. Catheter Cardiovasc Interv. 2016;87:467–475. doi: 10.1002/ccd.26137 [DOI] [PubMed] [Google Scholar]


