Abstract
Background
Cerebrovascular dysregulation syndromes, posterior reversible encephalopathy syndrome (PRES) and reversible cerebral vasoconstriction syndrome (RCVS), are challenging to diagnose because they are rare and require advanced neuroimaging for confirmation. We sought to estimate PRES/RCVS misdiagnosis in the emergency department and its associated factors.
Methods and Results
We conducted a retrospective cohort study of PRES/RCVS patients using administrative claims data from 11 states (2016–2018). We defined patients with a probable PRES/RCVS misdiagnosis as those with an emergency department visit for a neurological symptom resulting in discharge to home that occurred ≤14 days before PRES/RCVS hospitalization. Proportions of patients with probable misdiagnosis were calculated, characteristics of patients with and without probable misdiagnosis were compared, and regression analyses adjusted for demographics and comorbidities were performed to identify factors affecting probable misdiagnosis. We identified 4633 patients with PRES/RCVS. A total of 210 patients (4.53% [95% CI, 3.97–5.17]) had a probable preceding emergency department misdiagnosis; these patients were younger (mean age, 48 versus 54 years; P<0.001) and more often female (80.4% versus 69.3%; P<0.001). Misdiagnosed patients had fewer vascular risk factors except prior stroke (36.3% versus 24.2%; P<0.001) and more often had comorbid headache (84% versus 21.4%; P<0.001) and substance use disorder (48.8% versus 37.9%; P<0.001). Facility‐level factors associated with probable misdiagnosis included smaller facility, lacking a residency program (62.2% versus 73.7%; P<0.001), and not having on‐site neurological services (75.7% versus 84.3%; P<0.001). Probable misdiagnosis was not associated with higher likelihood of stroke or subarachnoid hemorrhage during PRES/RCVS hospitalization.
Conclusions
Probable emergency department misdiagnosis occurred in ≈1 of every 20 patients with PRES/RCVS in a large, multistate cohort.
Keywords: diagnostic error, emergency medicine, posterior reversible encephalopathy syndrome, reversible cerebral vasoconstriction syndrome, stroke
Subject Categories: Cerebrovascular Disease/Stroke
Nonstandard Abbreviations and Acronyms
- HCUP
Healthcare Cost and Utilization Project
- PRES
posterior reversible encephalopathy syndrome
- RCVS
reversible cerebral vasoconstriction syndrome
Clinical Perspective.
What Is New?
In a population‐level cohort study of patients hospitalized for posterior reversible encephalopathy syndrome/reversible cerebral vasoconstriction syndrome, nearly 5% had a probable preceding emergency department misdiagnosis.
Probable emergency department misdiagnosis of posterior reversible encephalopathy syndrome/reversible cerebral vasoconstriction syndrome was not significantly associated with the assessed markers of adverse clinical outcomes during subsequent posterior reversible encephalopathy syndrome/reversible cerebral vasoconstriction syndrome hospitalization.
What Are the Clinical Implications?
Increased suspicion for posterior reversible encephalopathy syndrome/reversible cerebral vasoconstriction syndrome in young emergency department patients presenting with neurological symptoms, particularly headache, may be warranted.
Over the past few years, several cohort studies have characterized posterior reversible encephalopathy syndrome (PRES) 1 , 2 and reversible cerebral vasoconstriction syndrome (RCVS), 3 , 4 leading to an increased recognition of these 2 entities as cerebrovascular dysregulation syndromes with overlapping presenting symptoms and a shared pathophysiology of transiently impaired cerebral autoregulation. 5 Despite our improved understating of these diseases, diagnosing PRES or RCVS is complicated by the relative rarity of these conditions, the need for advanced neuroimaging for confirmation, and the lack of well‐established diagnostic criteria for PRES/RCVS. 6 , 7 , 8 , 9 Rates of misdiagnosis of these cerebrovascular dysregulation syndromes are not well quantified, though rates of misdiagnosis of other cerebrovascular diseases in the emergency setting range from 3% to 9%. 10 , 11 Understanding current patterns of acute misdiagnosis among patients with PRES/RCVS is an essential first step toward improving diagnostic quality and safety for these patients. 12 , 13 We therefore sought to evaluate rates of probable PRES/RCVS misdiagnosis in the emergency department (ED) at the population level. We also sought to identify patient‐ and facility‐level factors associated with misdiagnosis as well as to assess the impact of a probable ED misdiagnosis on subsequent clinical outcomes.
Methods
We followed the Strengthening the Reporting of Observational Studies in Epidemiology guidelines. 14 The data that support the findings of this study are publicly available from the Healthcare Cost and Utilization Project (HCUP) at https://hcup‐us.ahrq.gov.
Design
We conducted a retrospective cohort study of patients with PRES, RCVS, or both using deidentified all‐payer claims data from all nonfederal EDs and hospitals across 11 states. These data were obtained from the Agency for Healthcare Research and Quality and its HCUP database. The included states were Arkansas, Florida, Georgia, Iowa, Massachusetts, Maryland, Nebraska, New York, Utah, Vermont, and Wisconsin. These 11 states were chosen because they are the only ones that provide contemporary longitudinal data to HCUP, thereby enabling tracking of patients across multiple ED visits and hospital admissions using an anonymous personal linkage number. 15 This study was conducted from 2016 to 2018. The Weill Cornell Medicine Institutional Review Board approved this study and granted a waiver of informed consent.
In this study, we used the National Academies of Sciences, Engineering, and Medicine definition of diagnostic error as a failure to establish an accurate and timely explanation of a patient's health problem. 12 In keeping with prior research, 16 we defined a probable misdiagnosis of PRES/RCVS as when (1) an ED visit resulted in discharge to home (treat‐and‐release visit), (2) the primary ED discharge diagnosis was for a neurological symptom (eg, headache, dizziness, numbness) that could represent an early manifestation of PRES/RCVS, and (3) the ED discharge occurred in the 14 days before the PRES/RCVS hospitalization. We defined a possible ED misdiagnosis as any ED treat‐and‐release visit during the 14 days before a PRES/RCVS hospitalization because PRES/RCVS can initially manifest with systemic complaints (eg, elevated blood pressure). 2 , 4 All other patients were considered as not having evidence of ED misdiagnosis (not misdiagnosed); patients with a cerebrovascular event during the 14 days before PRES/RCVS hospitalization were also considered not misdiagnosed, as these events can coincide with PRES/RCVS.
Measuring rates of diagnostic error or misdiagnosis using 2 points in time by pairing nonspecific symptoms initially thought to be benign with an unexpected adverse health event, as we do in this current study, is known as the Symptom–Disease Pair Analysis of Diagnostic Error conceptual approach. 17 This approach has been previously employed to measure rates of delayed cerebrovascular diagnoses using administrative claims data. 10 , 16 , 18 Here, our direction of analysis is from hospitalization for PRES/RCVS to a preceding ED treat‐and‐release visit for a related symptom(s), which constitutes a look‐back Symptom–Disease Pair Analysis of Diagnostic Error design. 17 Using visits resulting in discharge to home to evaluate for potential diagnostic error is a popular strategy that relies on the presumption that patients sent home from the ED are those who are thought, at the time of their ED evaluation, to have a low‐risk or benign condition that can be managed in the outpatient setting.
Population
To identify hospitalized patients with PRES/RCVS, we used validated International Classification of Diseases, Tenth Revision, Clinical Modification (ICD‐10‐CM) codes I67.841 and I67.83. A previous multicenter chart review study found a sensitivity of 100% (95% CI, 82–100) and specificity of 90% (95% CI, 79–96) for the diagnostic code I67.841 to detect RCVS. 7 Similarly, the diagnostic code I67.83 for PRES was previously found to have a sensitivity of 100% (95% CI, 86.8–100) and a specificity of 88.2% (95% CI, 72.6–96.7). 19
Measurements
We defined ED treat‐and‐release visits for neurological symptoms as those with a primary ICD‐10‐CM discharge diagnosis code for any neurological issue (eg, headache, numbness, dizziness, or altered mental status). ED visits in which a cerebrovascular condition was diagnosed were defined using well‐established ICD‐10‐CM codes. 20 ED treat‐and‐release visits for a nonneurological symptom were defined as those with a primary ICD‐10‐CM discharge diagnosis code for any nonneurological issue (eg, chest pain, vomiting).
We used ICD‐10‐CM codes recorded at the time of PRES/RCVS hospitalization to measure the following vascular comorbidities: hypertension, diabetes, coronary artery disease, congestive heart failure, atrial fibrillation/flutter, chronic obstructive pulmonary disease, chronic kidney disease, renal failure, and prior stroke. 21 , 22 , 23 We also used ICD‐10‐CM codes recorded at the time of PRES/RCVS hospitalization to measure the following comorbidities and triggers previously associated with PRES/RCVS: primary headache disorder, substance‐use disorder, drug poisoning, psychiatric illness, lupus, rheumatological disorders, cancer, and pregnancy/puerperium. 5
To explore the impact of probable misdiagnosis on patients' outcomes, we determined the frequency of ischemic stroke, intracerebral hemorrhage, and subarachnoid hemorrhage (SAH) using highly reliable ICD‐10‐CM diagnostic codes recorded at the PRES/RCVS hospitalization. 20 We also calculated length of stay for PRES/RCVS hospitalization and recorded discharge disposition.
We used data from the American Hospital Association to determine each facility's bed size, teaching status, and availability of neurological services.
Statistical Analysis
Standard descriptive statistics were used to characterize the study population, including means with SDs for normally distributed continuous variables and medians with interquartile ranges for nonnormally distributed continuous variables. The modified Wald method was used to determine CIs around our calculated proportions for misdiagnosis. To account for patients who had >1 preceding ED visit within 14 days of their PRES/RCVS hospitalization, we weighted patient‐ and facility‐level factors using visit frequency to identify factors associated with probable ED misdiagnosis. We compared patients with PRES/RCVS with a probable ED misdiagnosis to those without a misdiagnosis (not misdiagnosed). We also compared the ED facilities in which patients with a probable misdiagnosis had their treat‐and‐release visit(s) to the ED facilities in which patients without a probable misdiagnosis were seen. No adjustments were made for clustering of patients within a given ED.
Comparisons between groups were performed using the t test for continuous variables and χ2 test for categorical variables. To evaluate the joint influence of patient and facility factors on probable ED misdiagnosis, we constructed a multivariable model where all factors significantly associated with misdiagnosis in univariate analyses were included as predictors. We used multiple logistic regression to assess the relationship between probable ED misdiagnosis and subsequent (at the PRES/RCVS hospitalization) stroke as well as discharge disposition (discharged to home versus all other destinations) using odds ratios (ORs). To perform robust regression inference, sandwich standard errors were used. Linear regression was used to evaluate length of stay during PRES/RCVS hospitalization between patients with versus without a probable ED misdiagnosis. We adjusted all models for patient demographics and comorbidities. We used linear regression and graphed a histogram to evaluate the timing of ED treat‐and‐release visits before PRES/RCVS hospitalization among patients with a probable misdiagnosis. The threshold for statistical significance was set as <0.05. All tests of comparison were 2‐sided. Analyses were performed using Stata/MP, version 15.1 (StataCorp, College Station, TX). All missing data elements are explicitly acknowledged; we did not impute any data. Cenai Zhang had full access to all the data in the study and takes responsibility for their integrity and the data analysis.
Results
We identified 4633 patients hospitalized for PRES/RCVS; most (n=4169; 90.0%) had PRES. A total of 210 patients (4.5% [95% CI, 4.0–5.2]) had a probable ED misdiagnosis. A total of 615 patients (13.3% [95% CI, 12.3–14.3) had a possible ED misdiagnosis.
Among the 210 patients with a probable misdiagnosis, there were 281 ED treat‐and‐release visits in the 14 days before their PRES/RCVS hospitalization. There were 50 patients with a probable misdiagnosis who had >1 ED treat‐and‐release visit before their PRES/RCVS hospitalization; the median number of preceding ED visits was 1 (interquartile range, 1–2). The 3 most common ED treat‐and‐release visits' primary diagnoses among patients with a probable misdiagnosis were headache, migraine headache, and unspecified altered mental status. ED visit frequency increased closer in time to the PRES/RCVS hospitalization; there were 51 relevant ED visits ≤1 day before PRES/RCVS hospitalization as opposed to only 17 visits on the 14th day before PRES/RCVS hospitalization (P<0.001; Figure).
Figure 1. Pattern of emergency department treat‐and‐release visits over time among patients with a probable misdiagnosis of PRES/RCVS.
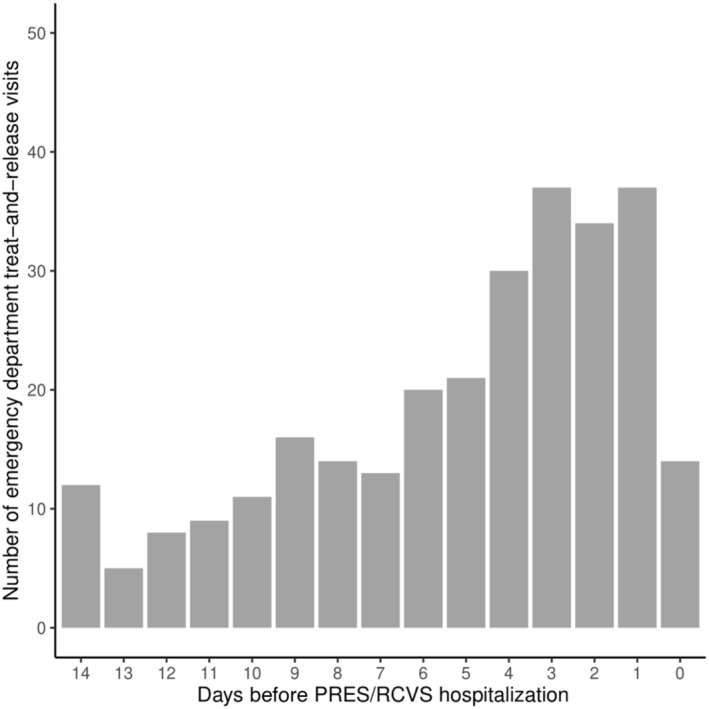
Emergency department treat‐and‐release visits for neurological symptoms; day 0 corresponds to the day of PRES/RCVS hospitalization. PRES/RCVS indicates posterior reversible encephalopathy syndrome/reversible cerebral vasoconstriction syndrome.
Among the 210 patients with PRES/RCVS with a probable misdiagnosis, 120 (57.14%) had a final diagnosis of PRES only, and 84 (40%) had a final diagnosis of RCVS only. As shown in Table 1, patients with a probable misdiagnosis were younger (47.7 years versus 54 years; P<0.001), more often women (80.4% versus 69.3%; P<0.001), and more often had private insurance as compared with those correctly diagnosed. Vascular comorbidities were all significantly more common in patients correctly diagnosed except for history of prior stroke, which was more common in those with a probable misdiagnosis (36.3% versus 24.2%; P<0.001). Patients with a probable misdiagnosis more often had a comorbid headache condition (84% versus 21.4%; P<0.001) and substance use disorder (48.8% versus 37.9%; P<0.001). Having a history of cancer (5% versus 13.1%; P<0.001), rheumatological disorder (4.3% versus 7.6%; P=0.041), including lupus, as well as being pregnant or postpartum were less common among patients with a probable misdiagnosis.
Table 1.
Differences Between Patients With Versus Without Probable Posterior Reversible Encephalopathy Syndrome/Reversible Cerebral Vasoconstriction Syndrome Misdiagnosis Weighted Using Visit Frequency
| No misdiagnosis (N=4018) | Probable misdiagnosis (N=281) | P value | |
|---|---|---|---|
| Demographics | |||
| Age, mean (SD), y | 54.0 (18.5) | 47.7 (15.1) | <0.001 |
| Sex, female, n (%) | 2783 (69.3) | 226 (80.4) | <0.001 |
| Race or ethnicity, n (%) | |||
| White | 2642 (65.8) | 207 (73.7) | 0.038 |
| Black | 884 (22.0) | 43 (15.3) | |
| Hispanic | 265 (6.6) | 18 (6.4) | |
| Other | 227 (5.7) | 13 (4.6) | |
| Primary expected payer, n (%) | |||
| Medicare | 1832 (45.6) | 67 (23.8) | <0.001 |
| Medicaid | 823 (20.5) | 71 (25.3) | |
| Private insurance | 1076 (26.8) | 123 (43.8) | |
| Other | 287 (7.1) | 20 (7.1) | |
| Vascular comorbidities, n (%) | |||
| Atrial fibrillation/flutter | 434 (10.8) | 11 (3.9) | <0.001 |
| Hypertension | 3420 (85.1) | 192 (68.3) | <0.001 |
| Diabetes | 1535 (38.2) | 66 (23.5) | <0.001 |
| Coronary heart disease | 998 (24.8) | 44 (15.7) | <0.001 |
| Congestive heart failure | 789 (19.6) | 27 (9.6) | <0.001 |
| Chronic kidney disease | 1377 (34.3) | 43 (15.3) | <0.001 |
| Renal failure | 1379 (34.3) | 43 (15.3) | <0.001 |
| Chronic obstructive pulmonary disease | 887 (21.8) | 39 (13.9) | 0.002 |
| Prior stroke | 947 (24.2) | 102 (36.3) | <0.001 |
| Additional comorbidities, n (%) | |||
| Benign headache | 861 (21.4) | 236 (84.0) | <0.001 |
| Substance use disorder | 1524 (37.9) | 137 (48.8) | <0.001 |
| Psychiatric illness | 1948 (48.5) | 142 (50.5) | 0.506 |
| Alcohol use | 390 (9.7) | 24 (8.5) | 0.804 |
| Drug poisoning | 1174 (29.2) | 70 (24.9) | 0.124 |
| Lupus | 154 (3.8) | * | 0.078 |
| Rheumatological disorders | 304 (7.6) | 12 (4.3) | 0.041 |
| Cancer | 527 (13.1) | 14 (5.0) | <0.001 |
| Pregnancy/puerperium | 227 (5.7) | * | 0.001 |
Counts suppressed to comply with privacy regulations regarding instances of <11 data points per cell.
In Table 2, facility factors associated with a probable ED misdiagnosis are reported. We found that probable misdiagnosis was associated with presenting to a facility without an Accreditation Council for Graduate Medical Education–approved residency program (62.2% versus 70.4%; P=0.006), to a facility without neurological services (75.7% versus 81.2%; P=0.032), and to a facility that was smaller in size. Due to a lack of hospital‐level identifiers for certain states in HCUP, facility‐level information was unavailable for 30 of 281 (10.7%) ED encounters among patients with a probable misdiagnosis and 717 of 4485 (16%) encounters among correctly diagnosed patients. In our multivariable model including both patient and facility factors, we found that comorbid headache at the time of PRES/RCVS hospitalization was most associated with probable ED misdiagnosis (OR, 15.21 [95% CI, 10.34–22.38]).
Table 2.
Facility‐Level Differences Between Emergency Department Encounters With Versus Without Probable Posterior Reversible Encephalopathy Syndrome/Reversible Cerebral Vasoconstriction Syndrome Misdiagnosis
| No misdiagnosis (N=3768) | Probable misdiagnosis (N=251) | P value | |
|---|---|---|---|
| Size based on total number of beds, n (%) | |||
| <100 | 288 (7.6) | 53 (21.1) | <0.001 |
| 100–499 | 1959 (52.0) | 131 (52.2) | |
| ≥500 | 1521 (40.4) | 67 (26.7) | |
| Accreditation Council for Graduate Medical Education–approved residency, n (%) | 2651 (70.4) | 156 (62.2) | 0.006 |
| Facility control, n (%) | |||
| Government | 405 (10.7) | 20 (8.0) | 0.223 |
| Nonprofit | 2860 (75.9) | 205 (81.7) | |
| For‐profit | 503 (13.4) | 26 (10.4) | |
| Neurological services at hospital | 3060 (81.2) | 190 (75.7) | 0.032 |
After adjustment for demographics and all comorbidities, probable misdiagnosis was not significantly associated with ischemic stroke (OR, 1.33 [95% CI, 0.75–2.35]), intracerebral hemorrhage (OR, 1.18 [95% CI, 0.57–2.45]), or SAH (OR, 1.28 [95% CI, 0.76–2.17]) documented during the PRES/RCVS hospitalization. Probable misdiagnosis was negatively related to length of stay such that the average length of stay was 2 days shorter for patients with a probable misdiagnosis after adjustment for demographics and comorbidities (P=0.003). There was no statistically significant association between probable misdiagnosis and discharge destination (OR, 0.71 [95% CI, 0.49–1.02]) after adjustment.
Discussion
In a large, heterogeneous cohort, we found that ≈1 of every 20 patients with PRES/RCVS had a probable misdiagnosis at a preceding ED visit. Probable misdiagnosis was more common among younger patients, women, and those with prior stroke, substance use disorder, or comorbid headache. We also found that preceding probable ED misdiagnoses more often occurred at hospitals that were smaller and without residency training programs or neurological services. Probable misdiagnosis in the ED was not significantly associated with a higher likelihood of ischemic stroke, intracerebral hemorrhage, or SAH during subsequent PRES/RCVS hospitalization.
The proportion of patients with PRES/RCVS estimated to have a preceding ED misdiagnosis is similar to diagnostic error rates reported with other uncommon cerebrovascular diseases, such as cerebral venous thrombosis (3.6%) 18 and cervicocephalic artery dissection (3.1%). 10 However, in a large study of patients hospitalized for more common cerebrovascular conditions (ischemic stroke, TIA, intracerebral hemorrhage, and SAH), rates of probable ED misdiagnosis were lower (1.2%). 16 Patients with rare cerebrovascular conditions thus may be at higher risk of ED misdiagnosis than those with more common ones. In our study, patients with a final diagnosis of RCVS more often had a preceding ED treat‐and‐release visit than those diagnosed with PRES. Increasing physician awareness and education about cerebral dysregulation syndromes or improving ED access to neurologists may be ways to improve PRES/RCVS diagnostic accuracy. As in our study, low‐volume and nonteaching hospitals without access to neurological consultation are facility‐level features that have previously been associated with diagnostic error. 16
Our results regarding patient factors associated with probable ED misdiagnosis of PRES/RCVS add to the growing literature identifying sex‐related differences in diagnosis among patients with cerebrovascular disease. 24 In a prior study of ED stroke misdiagnosis, male sex was associated with a lower odds of misdiagnosis 16 and, in a separate cohort study, women were underdiagnosed with TIA/minor stroke when they presented acutely with neurological symptoms. 25 Further research to improve diagnosis in women with PRES/RCVS and other cerebrovascular conditions is warranted and may represent an important opportunity to reduce disparities in cerebrovascular disease. Additionally, based on our study results, developing targeted strategies to improve diagnostic accuracy of PRES/RCVS among patients with a prior stroke or comorbid headache complaints may be useful. Such strategies may also be impactful for patients with other cerebrovascular diseases, as our prior work has similarly found that both patients with a history of stroke 26 and those with a history of headache 27 are at increased risk of missed and delayed diagnosis of cerebrovascular disease in the ED. Based on our study findings, increased suspicion for PRES/RCVS in young ED patients presenting with neurological symptoms may be warranted, especially in those for whom there may be a temptation to defer workup to the outpatient setting (eg, those with commercial insurance and easy access to outpatient care). While associations between substance abuse, mental health issues, and misdiagnosis of myocardial infarction in the ED have been previously found, 28 the ways in which substance use disorder may impact diagnostic accuracy in neurological disease require further study.
We did not find a statistically significant relationship between probable PRES/RCVS misdiagnosis and the adverse clinical outcomes we evaluated for in this study. Instead, we actually found that length of stay during PRES/RCVS hospitalization was significantly shorter for patients with a probable ED misdiagnosis. Based on prior studies, 10 , 29 a plausible explanation for our results regarding the effect of probable ED misdiagnosis on adverse outcomes is that many PRES/RCVS patients erroneously sent home from the ED had less severe disease or had fewer medical comorbidities than patients with PRES/RCVS who were initially hospitalized. Results from Symptom–Disease Pair Analysis of Diagnostic Error analyses must be interpreted carefully with the understanding that, when assessing the effect of misdiagnosis on clinical outcomes, what would have happened had a diagnostic error not occurred is of central importance, and this counterfactual is difficult to assess without detailed baseline prognostic data. 30 In the current study, our inability to determine the clinical severity of index ED presentations among included patients represents an important limitation of our results regarding adverse outcomes at PRES/RCVS hospitalization. While we did not find a significantly higher likelihood of stroke or SAH among patients with PRES/RCVS with a preceding ED misdiagnosis, patients who experienced ED misdiagnosis may still have been harmed by the error. 31
Our study has a number of additional limitations. To begin with, it is possible that we may have underestimated the rate of PRES/RCVS misdiagnosis in the ED because we did not capture patients who failed to return for medical evaluation after an ED visit, patients who were never correctly diagnosed with PRES/RCVS when they returned, and patients who died before an accurate diagnosis could be made. Alternatively, we may have overestimated rates of misdiagnosis as some ED treat‐and‐release visits in the 14 days before PRES/RCVS hospitalization may have been completely unrelated to cerebrovascular dysregulation. However, this latter possibility seems unlikely given the temporal relationships we found between encounters. Second, we may have overestimated the number of patients diagnosed with PRES/RCVS in a timely and accurate fashion because we considered any patients without an ED treat‐and‐release visit in the 14 days before PRES/RCVS hospitalization to be correctly diagnosed. It is possible that some of the patients we categorized as correctly diagnosed may have had a delayed diagnosis of PRES/RCVS during their hospitalization or may have been initially misdiagnosed at a non‐ED encounter (eg, outpatient office visit, urgent care visit) or initially presented to an ED in a state not included in our HCUP database. Third, given the relative rarity of probable ED misdiagnosis that we found, our study is likely underpowered. Fourth, missing information regarding facility‐level factors may have biased our results regarding which factors were associated with diagnostic error, as some states systematically lacked data regarding facilities. Finally, it is important to note that medication and imaging data were not available in HCUP, which limits our ability to fully characterize our study cohort.
Conclusions
Among patients hospitalized for PRES/RCVS, we found that ≈1 of every 20 patients were probably misdiagnosed at a preceding ED visit. While we identified certain patient‐ and facility‐level factors associated with ED misdiagnosis of PRES/RCVS, additional research is needed to identify targetable pathways to improve diagnostic accuracy among ED patients with these relatively rare but potentially disabling cerebrovascular disorders.
Sources of Funding
A.L. Liberman is supported by National Institute of Neurological Disorders and Stroke research grant K23NS10764. N.S. Parikh is supported by National Institute on Aging research grant K23AG073524 and the Florence Gould Foundation.
Disclosures
Dr Parikh is the site blinded outcome assessor for the Medtronic, A Study of the Embolization of the Middle Meningeal Artery With ONYXTM Liquid Embolic System In the Treatment of Subacute and Chronic Subdural HEmatoma (EMBOLISE) trial and has received personal fees for medicolegal consulting. Dr Kamel serves as a principal investigator for the National Institutes of Health–funded ARCADIA (Atrial Cardiopathy and Antithrombotic Drugs In Prevention After Cryptogenic Stroke) trial (National Institute of Neurological Disorders and Stroke u01ns095869), which receives in‐kind study drug from the BMS–Pfizer alliance for eliquis and ancillary study support from Roche Diagnostics; as deputy editor for JAMA Neurology; on clinical trial steering/executive committees for Medtronic, Janssen, and Javelin Medical; and on end point adjudication committees for Novonordisk and Boehringer–Ingelheim, not directly related to this work. Drs Merkler and Navi have received personal fees for medicolegal consulting. The remaining authors have no disclosures to report.
This manuscript was sent to Jose R. Romero, MD, Associate Editor, for review by expert referees, editorial decision, and final disposition.
For Sources of Funding and Disclosures, see page 7.
References
- 1. Bartynski WS, Boardman JF. Distinct imaging patterns and lesion distribution in posterior reversible encephalopathy syndrome. AJNR Am J Neuroradiol. 2007;28:1320–1327. doi: 10.3174/ajnr.A0549 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Fugate JE, Claassen DO, Cloft HJ, Kallmes DF, Kozak OS, Rabinstein AA. Posterior reversible encephalopathy syndrome: associated clinical and radiologic findings. Mayo Clin Proc. 2010;85:427–432. doi: 10.4065/mcp.2009.0590 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Singhal AB, Hajj‐Ali RA, Topcuoglu MA, Fok J, Bena J, Yang D, Calabrese LH. Reversible cerebral vasoconstriction syndromes: analysis of 139 cases. Arch Neurol. 2011;68:1005–1012. doi: 10.1001/archneurol.2011.68 [DOI] [PubMed] [Google Scholar]
- 4. Ducros A, Boukobza M, Porcher R, Sarov M, Valade D, Bousser MG. The clinical and radiological spectrum of reversible cerebral vasoconstriction syndrome. A prospective series of 67 patients. Brain. 2007;130:3091–3101. doi: 10.1093/brain/awm256 [DOI] [PubMed] [Google Scholar]
- 5. Singhal AB. Posterior reversible encephalopathy syndrome and reversible cerebral vasoconstriction syndrome as syndromes of cerebrovascular dysregulation. Continuum (Minneap Minn). 2021;27:1301–1320. doi: 10.1212/CON.0000000000001037 [DOI] [PubMed] [Google Scholar]
- 6. Fugate JE, Rabinstein AA. Posterior reversible encephalopathy syndrome: clinical and radiological manifestations, pathophysiology, and outstanding questions. Lancet Neurol. 2015;14:914–925. doi: 10.1016/S1474-4422(15)00111-8 [DOI] [PubMed] [Google Scholar]
- 7. Magid‐Bernstein J, Omran SS, Parikh NS, Merkler AE, Navi B, Kamel H. RCVS: symptoms, incidence, and resource utilization in a population‐based US cohort. Neurology. 2021;97:e248–e253. doi: 10.1212/WNL.0000000000012223 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Rocha EA, Topcuoglu MA, Silva GS, Singhal AB. RCVS2 score and diagnostic approach for reversible cerebral vasoconstriction syndrome. Neurology. 2019;92:e639–e647. doi: 10.1212/WNL.0000000000006917 [DOI] [PubMed] [Google Scholar]
- 9. Miller R, Wagner S, Hammond J, Roberts N, Marshall K, Barth B. Posterior reversible encephalopathy syndrome in the emergency department: a single center retrospective study. Am J Emerg Med. 2021;45:61–64. doi: 10.1016/j.ajem.2021.02.013 [DOI] [PubMed] [Google Scholar]
- 10. Liberman AL, Navi BB, Esenwa CC, Zhang C, Song J, Cheng NT, Labovitz DL, Kamel H, Merkler AE. Misdiagnosis of cervicocephalic artery dissection in the emergency department. Stroke. 2020;51:1876–1878. doi: 10.1161/STROKEAHA.120.029390 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Tarnutzer AA, Lee SH, Robinson KA, Wang Z, Edlow JA, Newman‐Toker DE. ED misdiagnosis of cerebrovascular events in the era of modern neuroimaging: a meta‐analysis. Neurology. 2017;88:1468–1477. doi: 10.1212/WNL.0000000000003814 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. National Academies of Sciences, Engineering, and Medicine . Improving Diagnosis in Health Care. The National Academies Press; 2015. doi: 10.17226/21794 [DOI] [Google Scholar]
- 13. Newman‐Toker DE, Schaffer AC, Yu‐Moe CW, Nassery N, Saber Tehrani AS, Clemens GD, Wang Z, Zhu Y, Fanai M, Siegal D. Serious misdiagnosis‐related harms in malpractice claims: the "big three"–vascular events, infections, and cancers. Diagnosis (Berl). 2019;6:227–240. doi: 10.1515/dx-2019-0019 [DOI] [PubMed] [Google Scholar]
- 14. von Elm E, Altman DG, Egger M, Pocock SJ, Gotzsche PC, Vandenbroucke JP, Initiative S. The strengthening the reporting of observational studies in epidemiology (STROBE) statement: guidelines for reporting observational studies. Lancet. 2007;370:1453–1457. doi: 10.1016/S0140-6736(07)61602-X [DOI] [PubMed] [Google Scholar]
- 15. Barrett M, Steiner C, Andrews R, Kassed C, Nagamine M; Methodological Issues when Studying Readmissions and Revisits Using Hospital Adminstrative Data . HCUP Methods Series Report # 2011–01. March 9, 2011. U.S. Agency for Healthcare Research and Quality. 2011. Accessed May 2, 2022. http://www.hcupus.ahrq.gov/reports/methods/methods.jsp.
- 16. Newman‐Toker DE, Moy E, Valente E, Coffey R, Hines AL. Missed diagnosis of stroke in the emergency department: a cross‐sectional analysis of a large population‐based sample. Diagnosis (Berl). 2014;1:155–166. doi: 10.1515/dx-2013-0038 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Liberman AL, Newman‐Toker DE. Symptom‐disease pair analysis of diagnostic error (SPADE): a conceptual framework and methodological approach for unearthing misdiagnosis‐related harms using big data. BMJ Qual Saf. 2018;27:557–566. doi: 10.1136/bmjqs-2017-007032 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Liberman AL, Gialdini G, Bakradze E, Chatterjee A, Kamel H, Merkler AE. Misdiagnosis of cerebral vein thrombosis in the emergency department. Stroke. 2018;49:1504–1506. doi: 10.1161/STROKEAHA.118.021058 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Parauda SC, Zhang C, Salehi Omran S, Schweitzer AD, Murthy SB, Merkler AE, Navi BB, Iadecola C, Kamel H, Parikh NS. Risk of stroke after posterior reversible encephalopathy syndrome. Stroke. 2022;53:3313–3319. doi: 10.1161/STROKEAHA.122.038673 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Kokotailo RA, Hill MD. Coding of stroke and stroke risk factors using international classification of diseases, revisions 9 and 10. Stroke. 2005;36:1776–1781. doi: 10.1161/01.STR.0000174293.17959.a1 [DOI] [PubMed] [Google Scholar]
- 21. Boehme AK, Esenwa C, Elkind MS. Stroke risk factors, genetics, and prevention. Circ Res. 2017;120:472–495. doi: 10.1161/CIRCRESAHA.116.308398 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Portegies ML, Lahousse L, Joos GF, Hofman A, Koudstaal PJ, Stricker BH, Brusselle GG, Ikram MA. Chronic obstructive pulmonary disease and the risk of stroke. The Rotterdam study. Am J Respir Crit Care Med. 2016;193:251–258. doi: 10.1164/rccm.201505-0962OC [DOI] [PubMed] [Google Scholar]
- 23. Masson P, Webster AC, Hong M, Turner R, Lindley RI, Craig JC. Chronic kidney disease and the risk of stroke: a systematic review and meta‐analysis. Nephrol Dial Transplant. 2015;30:1162–1169. doi: 10.1093/ndt/gfv009 [DOI] [PubMed] [Google Scholar]
- 24. Ali M, van Os HJA, van der Weerd N, Schoones JW, Heymans MW, Kruyt ND, Visser MC, Wermer MJH. Sex differences in presentation of stroke: a systematic review and meta‐analysis. Stroke. 2022;53:345–354. doi: 10.1161/STROKEAHA.120.034040 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Yu AYX, Penn AM, Lesperance ML, Croteau NS, Balshaw RF, Votova K, Bibok MB, Penn M, Saly V, Hegedus J, et al. Sex differences in presentation and outcome after an acute transient or minor neurologic event. JAMA Neurol. 2019;76:962–968. doi: 10.1001/jamaneurol.2019.1305 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Liberman AL, Hassoon A, Fanai M, Badihian S, Rupani H, Peterson SM, Sebestyen K, Wang Z, Zhu Y, Lipton RB, et al. Cerebrovascular disease hospitalizations following emergency department headache visits: a nested case‐control study. Acad Emerg Med. 2022;29:41–50. doi: 10.1111/acem.14353 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Liberman AL, Bakradze E, McHugh DC, Esenwa CC, Lipton RB. Assessing diagnostic error in cerebral venous thrombosis via detailed chart review. Diagnosis (Berl). 2019;6:361–367. doi: 10.1515/dx-2019-0003 [DOI] [PubMed] [Google Scholar]
- 28. Sharp AL, Pallegadda R, Baecker A, Park S, Nassery N, Hassoon A, Peterson S, Pitts SI, Wang Z, Zhu Y, et al. Are mental health and substance use disorders risk factors for missed acute myocardial infarction diagnoses among chest pain or dyspnea encounters in the emergency department? Ann Emerg Med. 2022;79:93–101. doi: 10.1016/j.annemergmed.2021.08.016 [DOI] [PubMed] [Google Scholar]
- 29. Ferro JM, Canhao P, Stam J, Bousser MG, Barinagarrementeria F, Massaro A, Ducrocq X, Kasner SE, Investigators I. Delay in the diagnosis of cerebral vein and dural sinus thrombosis: influence on outcome. Stroke. 2009;40:3133–3138. doi: 10.1161/STROKEAHA.109.553891 [DOI] [PubMed] [Google Scholar]
- 30. Newman‐Toker DE, Wang Z, Zhu Y, Nassery N, Saber Tehrani AS, Schaffer AC, Yu‐Moe CW, Clemens GD, Fanai M, Siegal D. Rate of diagnostic errors and serious misdiagnosis‐related harms for major vascular events, infections, and cancers: toward a national incidence estimate using the “big three.” Diagnosis (Berl). 2021;8:67–84. doi: 10.1515/dx-2019-0104 [DOI] [PubMed] [Google Scholar]
- 31. Newman‐Toker DE. A unified conceptual model for diagnostic errors: underdiagnosis, overdiagnosis, and misdiagnosis. Diagnosis (Berl). 2014;1:43–48. doi: 10.1515/dx-2013-0027 [DOI] [PMC free article] [PubMed] [Google Scholar]


